 |
 |
Zyklon-B
A Brief Report on the Physical Structure and Composition
by Harry W. Mazal OBE
Acknowledgment
This study was made possible through the generosity of Director Jerzy Wr�blewski of the Auschwitz-Birkenau State Museum in Oswiecim, Poland who kindly provided a small number of Zyklon-B pellets to the Holocaust History Project for physical and chemical analysis.1 Our thanks also to Dr. Franciszek Piper, Chief Historian; Teresa Swiebocka, Publications Director; and Wojciech Smolen, our guide and translator.
Introduction
Zyklon-B was a commercial rodenticide and pesticide in common use before World War II.2 The active lethal ingredient in the product is hydrogen cyanide, which is deadly to warm-blooded animals in very low concentrations, and to insects in considerably higher concentrations.3 DEGESCH (Deutsche Gesellschaft für Schädlingsbekämpfung mbH / German Vermin Controlling Company), a subsidiary of IG Farben 4, licensed two German companies for the manufacture and distribution of Zyklon-B: Tesch und Stabenow (Testa) and Heerdt-Lingler (Heli). Zyklon-B was also manufactured in one of its forms in the United States by the American Cyanamid Company 5 under license from the patent holders, I.G. Farben.
Hydrogen cyanide has historically been employed for the destruction of rodents and insect pests in granaries, orchards, warehouses, railway cars, cargo ships and submarines. 6. It was in common, indeed domestic, use in the United States during the latter part of the nineteenth century and the early part of the twentieth century7, although its preparation, storage and use was hazardous and inconvenient at that time.8. German scientists developed a method for adsorbing hydrogen cyanide gas onto various substrates [supports] and placing the resulting product in sealed steel cans. At the time of packaging the substrate and the gas were combined with a stabilizing chemical as well as with a strong-smelling and irritating warning agent.9. The resulting product was equally poisonous and as effective as ordinary hydrogen cyanide, but considerably more manageable. Its manufacturers received a patent and trademarked one of the presentations of the product as "Zyklon-B."
Various different substrates were selected for their ability to retain hydrogen cyanide for a certain, albeit brief, period of time after the cans were opened. Although hydrogen cyanide is a liquid up to just under 26� C, it has a very high vapor pressure and therefore gasifies rapidly even at -10� C, 10 a temperature far below its boiling point of 25.6� C. Selection of the right substrate for a particular application made it possible to control the out-gassing velocity of the poisonous gas to suit the circumstances.
This study does not intend to describe the out-gassing characteristics of each substrate as this has been amply discussed elsewhere.11. The purpose of this study is simply to identify the substrate used for the production of Zyklon-B as employed in the various gas chambers in Auschwitz and Birkenau for the purpose of murdering human beings.
Zyklon-B: Substrates
The literature12 and the publications mentioned earlier make it is possible to establish that Zyklon-B was produced with one of three substrates: (a) cardboard or "lignin" disks; (b) diatomaceous earth; and (c) "Erko" pellets. Peters describes these as "Pappescheiben, Kieselgur, and 'Erko' - Würfel." 13. The composition of the first two is clearly identified, but that of the third, 'Erko' pellets has remained the subject of considerable speculation. Pressac, in his monumental study14 describes 'Erko' variously as 'crystals' 15 and as 'porous silica known as Erko' 16, although he correctly identifies (a) as 'disks of ligneous fibre,' and (b) as 'Diagriess,' a trademark for Kieselgur or diatomaceous earth.
Many historians describe the Zyklon-B used in the gas and delousing chambers as being crystals or diatomaceous earth. 17 18 19 20 21 22, even though diatomaceous earth is invariably presented as a fine powder rather than 'crystals' or pellets. This has given rise to speculation by Holocaust deniers who seek out the smallest gap in any description in order to attack the body of an otherwise well-founded text.
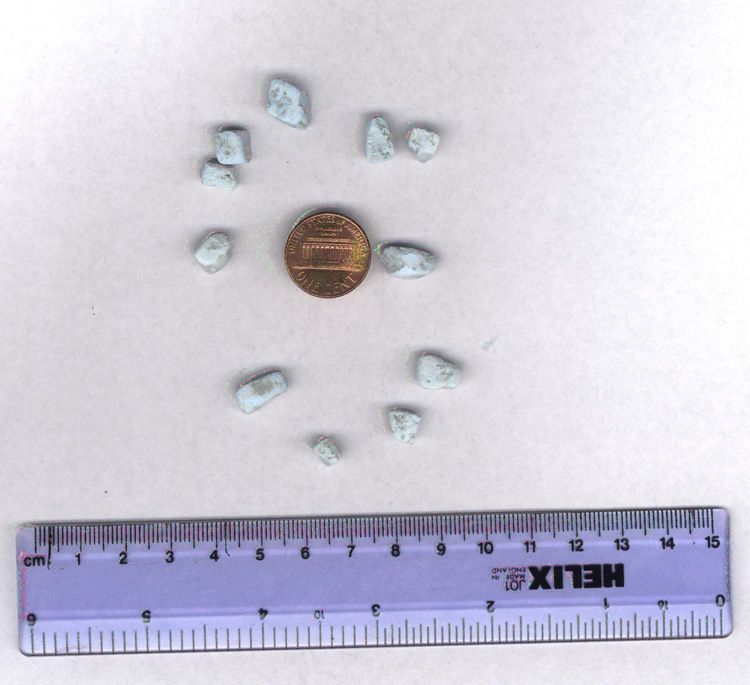
Zyklon-B pellets as used in the gas chambers in Auschwitz
Methodology
To put what might seem a trivial matter to rest, a request in writing was presented to the authorities at the Auschwitz Birkenau State Museum requesting that a small number of pellets of Zyklon-B be made available for research purposes. The pellets - no longer poisonous, one hastens to add - were given to the writer together with a covering letter on 10 July 2000. The pellets are small white cubes, roughly square or rectangular, 5 x 5 x 5 to 10mm in size, with a slight, bluish tinge, weighing under 500mg. They are chalky to the touch and appear to be quite porous, much like ordinary chalk.
Together with a sample of diatomaceous earth 23 and "Drierite" (anhydrous calcium sulfate)24, the Zyklon-B pellets were submitted to the Department of Electron Microscopy at the Universidad Autónoma Metropolitana in Mexico City. These three samples were identified simply as Samples 1, 2 and 3. This prestigious university was chosen in part because the equipment, a LEO Scanning Electron Microscope with an Energy Dispersive X-Ray Analysis accessory was sold and installed by the author's company.
All three samples were mounted on metal stubs and coated with a thin layer of gold in an Edwards High Vacuum evaporator. This is a standard procedure in scanning electron microscopy that eliminates a build-up of charges resulting from the electron beam. In all three cases the electron beam voltage was set at 20 KV. Several exposures were made of each sample with varying amplifications. For the purpose of this study three of the pictures made with the SEM were selected: Sample 1 was magnified 10,000X; Sample 2, 2,500X; and Sample 3 was magnified 3,500X.
The results are dramatic:
Sample 1, consisting of anhydrous calcium sulfate (Drierite) is totally amorphous even at relatively high magnifications. It has a remarkably small pore size, smaller than 1 micrometer.
![]()
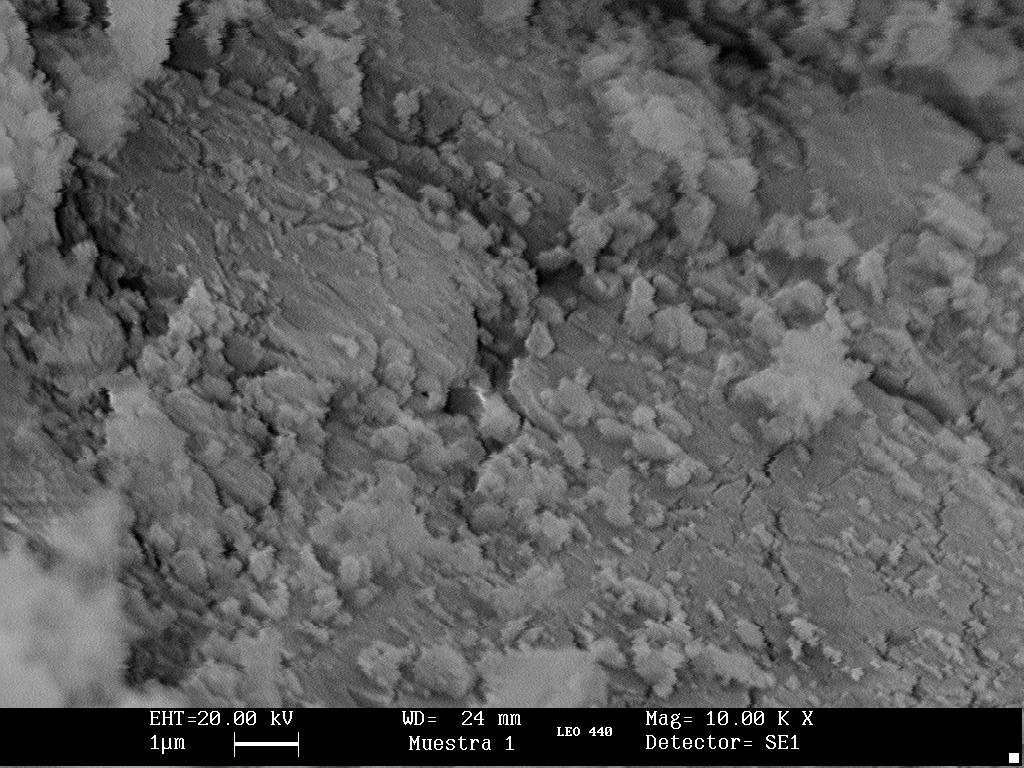
Sample 2, consisting of a Zyklon-B pellet, clearly shows a microcrystalline structure with orthorhombic crystals that are approximately 1.5 micrometers wide and 7 to 15 micrometers long. The pore size is several micrometers in diameter.
![]()
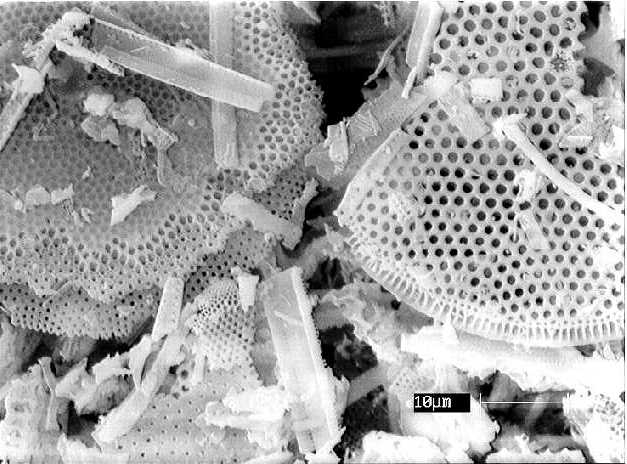
Sample 3, consisting of diatomaceous earth (Celite, Kieselgur, Diagriess) clearly shows the fossilized skeletons of diatoms that are approximately 40 micrometers in diameter.
![]()
From the scanning electron micrographs it can readily be established that Zyklon-B, as used in the Nazi camps, is not composed of diatomaceous earth. It is not possible simply from the image, however, to establish its chemical composition. The known Sample 1 is calcium sulfate but its amorphous structure is not even remotely similar to the micro-crystals of Sample 2.
To clear this remaining doubt, the samples were further analyzed using an Energy Dispersive X-Ray Analysis [EDX] accessory mounted on the Scanning Electron Microscope. The device permits accurate qualitative and semi-quantitative elemental analysis of inorganic materials. Conventional EDX can identify elements in compounds or mixtures from atomic weights from at least 12 upwards.
Here again the results are impressive:
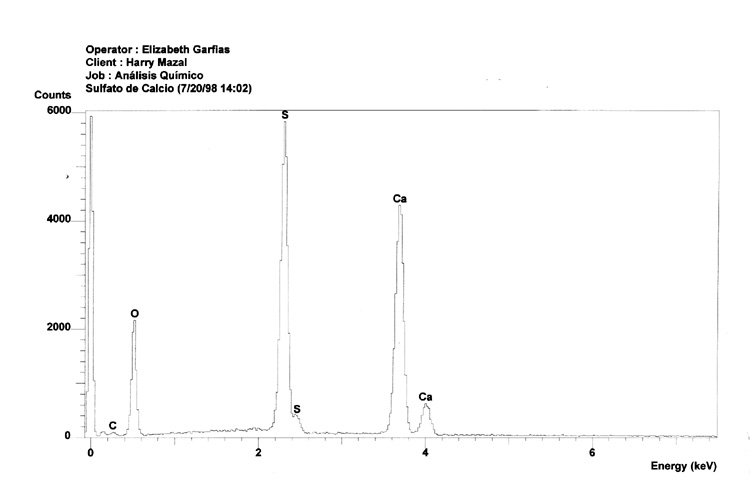
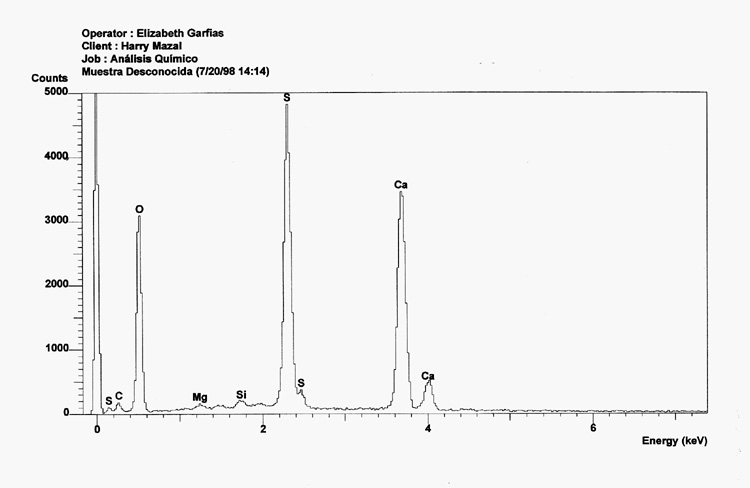
Sample 1 clearly shows clear peaks for Calcium, Oxygen and Sulfur. This is expected because the sample is composed of pure calcium sulfate in the form of the soluble anhydrite. This particular form of the mineral does not have a crystalline structure, 25 but does have a very small pore size.
![]()
Sample 2 equally shows clear peaks for Calcium, Oxygen and Sulfur, also showing some very minor traces of Barium and Aluminum. It is also calcium sulfate but is not the soluble anhydride given its crystalline structure.26
![]()
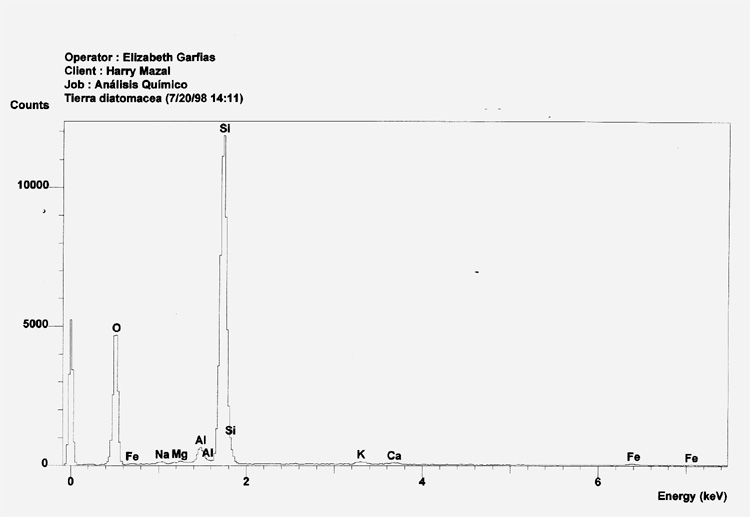
Sample 3 shows strong peaks for Silicone and Oxygen, with minor traces of Potassium, Iron, Calcium and Aluminum. This is as expected because diatomaceous earth is almost pure silicone dioxide. 27
![]()
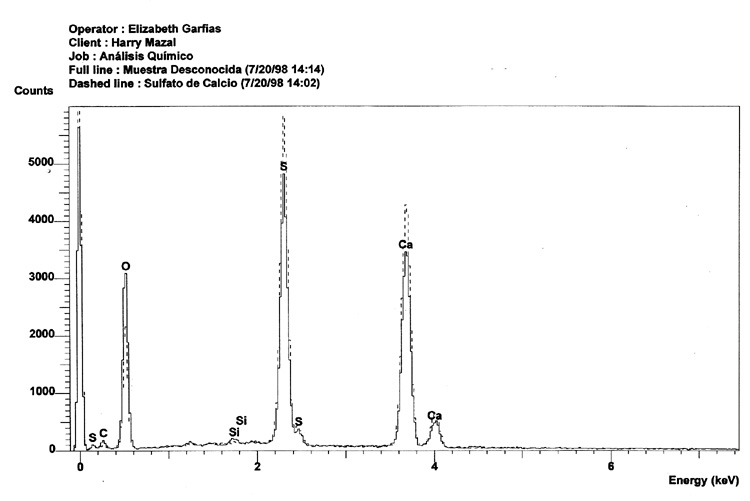
A comparison between the EDX analysis of a Zyklon-B pellet and a sample of calcium sulfate clearly demonstrates that they are identical in their composition. Note that the solid line is the "unknown"sample, or Zyklon-B, and that the dashed line is Calcium Sulfate.
![]()
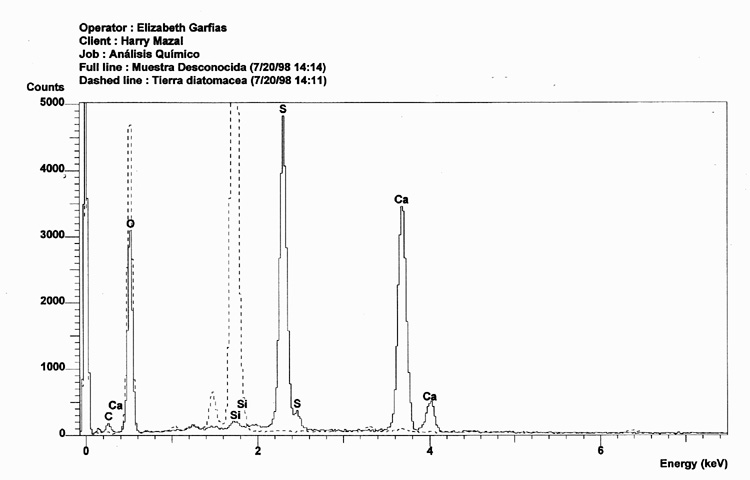
A comparison between the EDX analysis of a Zyklon-B pellet (solid line), and that of Diatomaceous Earth (dashed line) clearly demonstrates that Zyklon-B is not composed of Diatomaceous Earth.
![]()
Conclusions
It can be established that:
- Diatomaceous earth is composed of silicone dioxide: SiO2
- The pellets of Zyklon-B stored in the Auschwitz Birkenau State Museum are not made from diatomaceous earth.
- Zyklon-B is composed of calcium sulfate but not the soluble anhydrite of calcium sulfate.
- Due to its microcrystalline structure it is possible to determine that the Zyklon-B sample is either the natural form of anhydrous calcium sulfate, also known as the mineral anhydrite or, equally likely, the insoluble anhydrite resulting from heating gypsum at temperatures above 650 degrees centigrade. Both of these forms preserve the microcrystalline structure.28
Notes
- Letter dated 10 July 2000 from mgr Jerzy Wróblewski, Director of the Panstwowe Museum Auschwitz-Birkenau, to the Holocaust History Project, enclosing ten pellets of Zyklon-B.
- Peters, G., Blausäure zur Schädlingsbekämpfung, pp. 49/50, 1933, Verlag Von Ferdinand Enke (Stuttgart). Available in German at: http://www.holocaust-history.org/works/peters-1933/htm/intro00.htm
- Lewis, Robert A., Lewis' Dictionary of Toxicology, p. 578, 1998, CRC Press (Boca Raton)
- Hayes, Peter, Industry and Ideology: IG Farben in the Nazi Era, pp. 361-62, 1987, Cambridge University Press (Cambridge, England)
- American Cyanamid and Chemical Corporation.
Military Fumigation Manual: Zyklon Discoids for Insect Control. 1944 - Hiscox, Gardner D. (Editor), Henly's Formulas for Home and Workshop, pp. 418-419, "The Use of Hydrocyanic Gas for Exterminating Household Insects", 1979, Avenel Books (New York)
- Peters, G., Blausäure zur Schädlingsbekämpfung, pp. 37-49
- Peters, G., Blausäure zur Schädlingsbekämpfung, pp. 49-56
- Peters, G., Blausäure zur Schädlingsbekämpfung, p.59
- Peter(s), G. and Rasch W., Die Einsatzfähigkeit der Blausäure-Durchgasung bei tiefen Temperaturen, (Praktische Erfahrungen des Kriegswinters 1940/41 und ihre Nachpr�fung), Deutsche Gesellschaft für Schädlingsbekämpfung. Available in German and English at: http://www.holocaust-history.org/works/peters-rasch-1941/htm/intro000.htm
- Irmscher, R., Nochmals: Die Einsatzfähigkeit der Blausäure bei tiefen Temperaturen, (Deutsche Gesellschaft für Schädlingsbekämpfung), 1942. Available in German and English at: http://www.holocaust-history.org/works/irmscher-1942/htm/intro000.htm
- Nuremberg Document NI-9912, "Directive for the Use of Prussic Acid (Zyklon) for the Destruction of Vermin (Disinfestation): II Method of Using Prussic Acid."
- Peters, G., Blausäure zur Schädlingsbekämpfung, p. 60
- Pressac, Jean Claude, Auschwitz: Technique and Operation of the Gas Chambers, 1989, Beate Klarsfeld Foundation (New York)
- Pressac, Jean Claude, Auschwitz: Technique and Operation of the Gas Chambers, p. 15
- Pressac, Jean Claude, Auschwitz: Technique and Operation of the Gas Chambers, p. 21
- Bauer, Yehuda, A History of the Holocaust, p.215, 1982, Franklin Watts (New York)
- Gutman, Yisrael and Berenbaum, Michael. Editors, Anatomy of the Auschwitz Death Camp, p. 167. "Gas Chambers and Crematoria", Francizek Piper. 1994, Indiana University Press (Bloomington)
- Gutman, Israel, Editor. Encyclopedia of the Holocaust, Vol. IV, p. 1749. 1990, Macmillan Publishing Company (New York).
- Reitlinger, Gerald. The Final Solution: The Attempt to Exterminate the Jews of Europe 1939-1945, p. 151. 1987, Jason Aronson, Inc. (Northvale, New Jersey).
- Kogon, Eugen, Langbein, Herman and R�ckerl, Adalbert (Editors). Nazi Mass Murder: A Documentary History of the Use of Poison Gas. p. 206. 1994, Yale University Press (New Haven)
- Dwork, Debórah and van Pelt, Robert Jan. Auschwitz: 1270 to the Present. p. 293. 1996, W. W. Norton & Company (New York).
- Diatomaceous Earth, acid washed, not further calcined. Approx. 95% SiO2 60 mesh, Lot 97H3640, Sigma Chemical Co., P.O. Box 14508, Saint Louis, Missouri 63178
- "Drierite" Anhydrous Calcium Sulfate, 100% CaSO4 4 mesh, Lot CAS#7778-18-9, W. A. Hammond Drierite Company, Ltd., Xenia, Ohio 45385
- Budavari, Susan (Editor) The Merck Index Eleventh Edition, p. 1714, 1989, Merck & Co., Inc. (Rahway N.J.): "Soluble anhydrite is obtained in granular or powder form by complete dehydration of gypsum at below 300� in an electric oven. Estimated pore size is 38% by volume. Possesses high affinity for water and will absorb 6.6% of its weight forming the stable hemihydrate.
- Budavari, Susan (Editor) The Merck Index Eleventh Edition, p. 1714: "The natural form of anhydrous calcium sulfate is known as the mineral anhydrite; also as karstenite, muriacite, anhydrous sulfate of lime, anhydrous gypsum. Crystals are orthorhombic, color varies, e.g. white with blue, gray or reddish tinge, or brick red.
- Budavari, Susan (Editor) The Merck Index Eleventh Edition, p. 4878: "Infusorial Earth. diatomaceous earth; fossil flour; kieselguhr; Celite; Super-Cel. Siliceous frustules and fragments of various species of diatoms. See also Silicon Dioxide. White to light gray to buff powder. Insol. in water, acid or dil. alkalis. Capable of taking up and holding about 4 times its wt of water."
- Budavari, Susan (Editor) The Merck Index Eleventh Edition, p. 1714: "Insoluble anhydrite or dead-burned gypsum which has the same crystal structure as the mineral is obtained upon complete dehydration of gypsum at above 650�."