|
Reducing
the Risk for Cardiovascular Disease with Nutritional Supplements Matthias Rath, M.D.
|
|||
|
|
Abstract. Reducing the risk for cardiovascular disease (CVD)
is a primary goal of any health care system in the industrialized world. The success
of this world-wide effort will largely depend on the proper understanding of
the mechanisms responsible for development of this disease. This
paper marshals the scientific evidence for the predominant pathomechanisms of
CVD and presents new therapeutic approaches. Human atherosclerotic lesions
are primarily composed of lipoprotein(a). The extracellular deposition of
this lipoprotein directly parallels the extent of the atherosclerotic lesion.
The frequency of this pathomechanism today is directly related to its
efficacy as a defense mechanism during the evolution of man, particularly in
stabilizing the vascular wall during ascorbate deficiency. The deposition of
lipoprotein(a) in form of largely intact particles implies the reversibility
of this mechanism. On the
basis of an improved understanding about the pathogenesis of CVD new
therapeutic approaches are defined. Certain vitamins and amino acids are of
particular importance for these approaches. Ascorbate is essential for
preserving and restoring the integrity and stability of the vascular wall.
Niacin and ascorbate were reported to lower lipoprotein(a) plasma levels. It
is proposed that this effect is mediated by NADPH. The
amino acids L-lysine and L-proline competitively interfere with the binding
of lipoprotein(a) to constituents of the vascular wall and atherosclerotic
lesions. The therapeutic use of these amino acids could prevent further
buildup of lipoprotein(a) accumulation in the vascular wall. More
importantly, optimum concentrations of L-lysine and L-proline could release
deposited lipoprotein(a) but also other atherogenic lipoproteins form the
vascular wall. This
paper defines a new therapeutic goal: The pharmaceutical, non-invasive
reversal of existing CVD with nutritional supplements. |
|
|
|
Introduction Cardiovascular Disease
(CVD) is the most frequent cause of death in the industrialized world. In a series
of recent papers I have contributed to an improved understanding about the
pathogenesis of human CVD. It was shown that ascorbate deficiency is an
important underlying factor and that all mechanisms known today leading to
CVD can be triggered by ascorbate deficiency. This remarkable fact reflects
the strong pressure during the evolution of man after loss of endogenous
ascorbate synthesis. This pressure favored genetic and metabolic features
contributing to avoidance of the fatal consequences of ascorbate deficiency
and scurvy. The different mechanisms of human CVD known today therefore all
compensate for impaired integrity and stability of the vascular wall caused
by chronically low dietary ascorbate intake. If these mechanisms overshoot,
heart attack, stroke and other forms of CVD develop. 1,2 In the first part of this
paper I will marshal the evidence for the most frequent of these
pathomechanisms. I will focus here on mechanisms related to lipid and
lipoprotein deposition in the vascular wall and re-evaluate existing
hypotheses. This re-evaluation is particularly necessary since cholesterol
lowering concepts have become dominant factors in the public health debate. I
will show that the most important among these overshooting defense mechanisms
is the extracellular deposition of lipoprotein(a) in the vascular wall. On
the basis of an improved understanding of these pathomechanisms I will
present new therapeutic approaches including the reversibility of existing
atherosclerotic deposits. Finally I will marshal the evidence for the
particular value of nutritional supplements to achieve this therapeutic aim. |
|
|
|
|
|
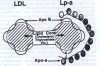
Lipoprotein(a), not LDL,
is the Primary Risk Factor for CVD in Plasma Present theories of human CVD are based on the concept that low-density lipoprotein (LDL) or LDL-cholesterol is the primary risk factor for CVD in plasma.3,4 A closer look at the available epidemiological data challenges this assumption. Lipoprotein(a), not LDL is the primary risk factor for CVD in human plasma. Lipoprotein(a) is a unique particle essentially composed of a LDL particle and an additional adhesive protein designated apoprotein(a) (apo(a)). The adhesive properties of apo(a) are the cause for the selective retention of lipoprotein(a) in the vascular wall and for the accumulation of lipids and lipoproteins inside the wall (Figure 1). Lipoprotein(a) is an independent risk factor for CVD. None of the epidemiological studies thus far assessing the plasma risk profile for CVD showed any correlation between lipoprotein(a) levels and total-cholesterol or LDL-cholesterol levels. The most conclusive study that lipoprotein(a), not LDL, is the primary risk factor for CVD was carried out in a genetically defined cohort of LDL-receptor deficient patients.5 This genetic disorder is characterized by significantly elevated plasma LDL levels and was thought to lead almost invariably to premature CVD. Surprisingly, 60% of these LDL-receptor deficient patients had no clinical signs of CVD, while 40% had developed CVD. Both groups did not differ in their extremely high plasma levels of LDL-cholesterol (above 300 mg/dl) or of total cholesterol (390 mg/dl). The two groups differed, however, significantly in their lipoprotein(a) plasma levels and CVD patients had on average three-fold higher plasma lipoprotein(a) levels. This study in a large group of patients selected to minimize genetic variations allows the following conclusions:
Equally strong evidence that lipoprotein(a), not LDL, is
the primary risk factor for CVD comes from a recent re-evaluation of the
Framingham Heart Study, one of the largest prospective epidemiological
studies determining the risk profile for CVD. Lipoprotein(a) ranked among the
most prevalent risk factors for heart attacks. Moreover, a given quantity of
lipoprotein(a) in the blood conferred as much added risk for CVD as does 10
times the quantity of LDL.6 Lipoprotein(a) was discovered 30 years
ago.7 The negligent exclusion of this important risk factor from previous
epidemiological studies deserves an explanation. It may in part be provided
by methodological difficulties as a result of the structural similarity
between lipoprotein(a) and LDL. Plasma lipoproteins in most epidemiological
studies were determined by means of the "Friedewald Formula",8 a
method that does not allow the differentiation between LDL and
lipoprotein(a). The re-evaluation of all large epidemiological risk factor
studies has become necessary. The results of these evaluations will further
confirm lipoprotein(a) as the primary risk factor for CVD. The evidence that
lipoprotein(a), not LDL, is the primary risk factor for CVD is not limited to
human plasma. |
|
|
|
Lipoprotein(a), not LDL, is the Primary Risk Factor
Contributing to Atherosclerotic Plaques Present concepts of human atherosclerosis assume that LDL is the main vehicle by which cholesterol and other lipids are deposited in the vascular wall. More recently it has been proposed that cellular uptake of oxidized LDL by macrophages and other scavenger cells and subsequent foam cell formation are the decisive mechanisms for development of atherosclerotic plaques.4 According to this concept foam cell formation or the extracellular deposition of LDL would have to play a decisive role in the progression of atherosclerotic lesions. A closer histological look on the in situ situation of human atherosclerotic lesions challenges this concept. The progression of atherosclerotic deposits is paralleled by a structural impairment of the vascular wall and by the accumulation of lipoprotein(a). Together with my colleagues at Hamburg University I reported the most comprehensive studies differentiating between the deposition of LDL and lipoprotein(a) in human atherosclerosis. ??¹¹ Although these studies are frequently quoted, their significance for the development of human atherosclerosis is still insufficiently understood. These studies and their correct interpretation have significant implications for future therapeutic approaches for CVD. The conclusions of these studies are marshaled here as follows:
|
|
|
|
|
|
1.
Lipoprotein(a) is the predominant risk factor contributing
to the progression of atherosclerotic lesions in man. 2.
The amount of lipoprotein(a) deposited in atherosclerotic
lesions corresponds with the extent of the lesions. 3.
Lipoprotein(a) is deposited in the extracellular matrix of
the vascular wall in the form of largely intact lipoprotein particles, which
can be isolated from the wall. This finding implies the reversibility of the
lipoprotein(a) deposition in the vascular wall. 4.
Isolated LDL deposition was rarely found and LDL alone,
without simultaneous lipoprotein(a) deposition, cannot be considered a
primary factor determining the advancement of human atherosclerotic lesions. 5.
The adhesive protein apo(a) is responsible for the
selective retention of the lipoprotein(a) particle inside the vascular wall
compared to LDL and other lipoproteins. These results do not exclude the deposition of other
potentially atherogenic lipoproteins (LDL, very low-density lipoprotein in
VLDL) in addition to and in the same areas lipoprotein(a)
accumulated. The discovery of the predominant role of lipoprotein(a) in human
atherosclerosis and the discovery of its potential reversibility were
decisive preconditions directly leading the way to identify the therapeutic
approaches discussed below. |
|
|
|
Mechanism Leading to the Extracellular Accumulation of
Lipoprotein(a) in the Vascular Wall The extracellular accumulation of lipoprotein(a) in the
vascular wall as the predominant pathomechanism of human atherosclerosis is
no coincidence. The frequency of this mechanism today is directly related to
its advantage during the evolution of man. After the loss of endogenous
ascorbate production in our ancestors lipoprotein(a) became a life-saving feature
to counteract fatal blood-loss through the scorbutic vascular wall. While
scurvy is essentially unknown today, chronic insufficient dietary ascorbate
intake is widespread. The deposition of lipoprotein(a) in the vascular wall
stabilizes the wall of the arteries particularly during ascorbate deficiency.
With insufficient dietary ascorbate intake over decades this defense
mechanism overshoots and CVD develops. 1,2 The lipoprotein(a) particle is an ideal defense molecule. Apo(a), an adhesive molecule,¹² interacts with a variety of cellular and extracellular constituents of the vascular wall including collagen, elastin, fibronectin, and glycosaminoglycanes as well as fibrin/ fibrinogen. The apo(a) macromolecule itself as well as the lipoprotein(a) particle confer stability to the structurally impaired vascular wall. Moreover, the deposition of lipoprotein(a) in the vascular wall can favor the additional retention of other lipoprotein particles such as LDL and VLDL. Lipoprotein(a) has been shown to bind to lipoproteins containing apoB¹³ and the accumulation of LDL and VLDL in addition to elevated lipoprotein(a) levels but not alone.5 With the extracellular deposition of lipoprotein(a) nature developed a sophisticated and reversible mechanism to render compensatory stability to the vascular wall during times when these walls ware weakened by a deficiency of essential nutrients. The reversible deposition of lipoproteins in the vascular wall is a key to new therapeutic approaches. To optimally exert this defense function the lipoprotein(a) particle would inevitably lead to a loss of its function to confer stability. In contrast to this mechanism, present hypotheses on human atherogenesis presuppose the degradation of the lipoprotein particles into lipids and amino acids by scavenging cells in the vascular wall.4 The importance of these mechanisms in the development of human atherosclerosis needs to be further evaluated. It is, however, evident that these mechanisms are inferior to the extracellular deposition of lipoprotein(a) with respect to two important features: stability and reversibility. This may explain why neither foam cell formation nor the extracellular deposition of LDL are found to parallel the progression of atherosclerotic lesions. Irrespective of the pathomechanisms of human atherogenesis
they can largely be prevented by maintaining the structural integrity,
stability, and elasticity of the vascular wall. On the basis of an improved
understanding of human CVD presented in the first part of this paper I will
now summarize the most important preventive and therapeutic aims for this
disease. |
|
|
|
|
|
Therapeutic Aim #1: Preserving and Restoring the Integrity and Stability of the
Vascular Wall The impairment of the vascular connective tissue and loss of the endothelial barrier functions are the underlying morphologic changes of any form of CVD. The instability of the vascular wall is a prominent risk factor for human CVD explaining the predominantly localized clinical manifestation of this disease in form of heart attack and stroke.¹'² Preserving and restoring the integrity and stability of the vascular wall is the most important therapeutic aim for prevention and treatment of human CVD. Integrity and stability of connective tissue are critically dependent on an optimum amount and function of collagen and elastin. Ascorbate stimulates the production of collagen and elastin and thereby directly contributes to preserving and restoring the stability and integrity of the vascular wall.14 It therefore comes as no surprise that CVD is essentially
unknown in animals producing their own vitamin C at a daily rate of several
thousand milligrams. Nor is it a surprise that lipoprotein(a) is primarily
found in species that had lost the ability of ascorbate synthesis, a
discovery I made in 1987. In humans a growing amount of clinical and
epidemiological data support the value of ascorbate in the prevention of CVD.
A recent epidemiological study in 11,000 Americans showed that dietary
ascorbate intake between 200 mg and 500 mg correlated with a reduction in CVD
up to 50% and an increase in life expectancy for up to 6 years.15
Beside providing structural stability to the human body, ascorbate is also
involved in a variety of enzymatic and other metabolic functions, some of
which will be discussed below. |
|
|
|
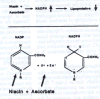
Therapeutic Aim #2: Lowering Lipoprotein(a) Levels in Plasma Lowering the plasma levels of lipoprotein(a) is the second most important therapeutic aim. Lipoprotein(a) is produced in the liver and the production rate of apo(a) largely determines the plasma levels of this lipoprotein. None of the currently available cholesterol-lowering drugs is known to significantly affect plasma lipoprotein(a) levels. In contrast, optimum dosages of two vitamins, niacin (vitamin B3) and ascorbate have been reported to lower lipoprotein(a) plasma levels.16-18 Their therapeutic mechanism, however, has not yet been explained. I have obtained preliminary in vitro evidence that lipoprotein(a) production can be lowered by increasing the concentration of NADPH. NADPH is involved in a multitude of metabolic regulatory processes. Niacin is a constituent of the NADP molecule and ascorbate can reduce or "re-charge" the NADP molecule to NADPH. Thus ascorbate and niacin could decrease lipoprotein plasma levels – at least in part – by increasing NADPH concentrations (Figure 2). Beside the lowering of lipoprotein(a) in plasma the risk
for CVD can be further reduced by preventing accumulation of this risk factor
in the vascular wall. |
|
|
|
|
|
Therapeutic Aim #3: Preventing the Accumulation of Lipoprotein(a) Prevention of the accumulation of lipoprotein(a) in the vascular wall is an important therapeutic aim in reducing the risk of CVD. As discussed above the lipoprotein(a) particle can interact with a variety of constituents of the vascular wall. The extracellular deposition of lipoprotein(a) particles in the vascular wall via the adhesive protein apo(a) immediately suggests novel therapeutic approaches. Interfering with the binding of lipoprotein(a) to constituents of the vascular wall will decrease the tendency of this atherogenic lipoprotein to accumulate in the vascular wall and thereby reduce the risk for the development of atherosclerotic lesions. The amino acids L-lysine, L-proline, and hydroxyproline can interfere with the binding of lipoprotein(a) to important constituents of the vascular wall.¹³'¹? The use of L-lysine and L-proline to prevent the deposition of atherogenic lipoproteins in the vascular wall opens novel therapeutic avenues. Supplementation of hydroxyproline and hydroxylysine can be rendered redundant by co-administration of ascorbate which can hydroxylate lysine and proline residues.² |
|
|
|
|
L-Lysine The essential amino acid L-lysine competitively inhibits the binding of lipoprotein(a) to fibrinogen, fibrin, and fibrin degradation products which are known to be hallmarks of the atherosclerotic lesion. My earlier findings about the potential reversibility of lipoprotein(a) deposition and the isolation of lipoprotein(a) by use of lysine led to the therapeutic introduction of L-lysine and lysine analogs in an earlier paper¹ (Figure 3a). |
|
|
|
|
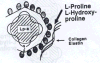
L-proline and hydroxyproline Trieu et al. Reported that lipoprotein(a) also binds to
L-proline and hydroxyproline with an even higher affinity than to lysine.¹³
Since collagen and elastin are particularly rich in proline residues this
mechanism is of importance for the binding and retention of the
lipoprotein(a) particle in the vascular wall. On the basis of these
observations I propose here the therapeutic use of L-proline in the
prevention and treatment of CVD. The dietary supplementation of this amino
acid should prevent the binding of lipoprotein(a) to collagen and other
proline-rich constituents of the vascular wall and thereby prevent the
accumulation of lipoprotein(a) in the vascular wall (Figure 3b). |
|
|
|
Therapeutic Aim #4: Reversal of Existing Atherosclerotic Lesions The improved understanding about human atherosclerosis and in particular about the role of lipoprotein(a) discussed in this paper opens the way to a break-through in the treatment of CVD: the pharmaceutical reversal of existing atherosclerotic lesions. The key to this breakthrough is the reversibility of the accumulation of lipoprotein(a) in the vascular wall. Through the same mechanism by which L-lysine and L-proline can prevent lipoprotein(a) deposition, optimum concentrations of these amino acids can release accumulated lipoprotein(a) from the vascular wall. The release of lipoprotein(a) from the atherosclerotic lesions must lead to a reduction of these atherosclerotic deposits and thereby to a reversal of existing CVD. Dietary supplementation of optimum amounts of L-lysine and
L-proline could contribute to releasing lipoprotein(a) deposited in the
vascular wall. The experimental evidence for these novel therapeutic options
is already available. Comprehensive clinical confirmation should soon lead to
the reduction of existing atherosclerotic deposits in CVD patients on the
basis of selected nutritional supplements. |
|
|
|
|
|
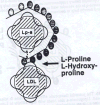
Therapeutic Aim #5: Reducing the Risk for CVD from Other Lipids and Lipoproteins LDL While the CVD risk for elevated LDL levels alone has to be re-evaluated, elevated LDL levels in addition to elevated lipoprotein(a) levels are known to increase the risk for CVD exponentially.5 This fact can be explained by the following mechanism. LDL can bind to lipoprotein(a) via proline residues (Figure 3c). This binding of LDL to lipoprotein(a) already deposited in the vascular wall can accelerate the development of atherosclerotic lesions. In the light of this mechanism, lowering elevated plasma
levels of LDL remains a therapeutic aim. In numerous studies niacin as well
as ascorbate have been shown to reduce elevated plasma levels of LDL. As with
lipoprotein(a) NADPH may play a regulatory role on the synthesis rate of VLDL
the precursor of LDL. Moreover, dietary supplementation of L-proline could prevent
the binding of LDL to lipoprotein(a) already deposited in the vascular wall
and, by the same mechanism, release already deposited LDL from the
atherosclerotic lesions. |
|
|
|
|
VLDL VLDL is a potentially atherogenic precursor of LDL particularly enriched in triglycerides. Niacin and ascorbate have also been shown to be of particular value in lowering VLDL plasma levels. Moreover, optimum L-proline concentrations should also interfere with the binding of VLDL inside the vascular wall. Thus dietary supplementation of ascorbate and niacin are of particular value to decrease the plasma levels of atherogenic lipoproteins. Optimum dietary supplementation with the amino acids L-lysine and L-proline could release not only lipoprotein(a) but also other atherogenic lipoproteins from the vascular wall. VLDL and other triglyceride-rich lipoproteins, however,
can contribute to atherogenesis also by another mechanism. Their enrichment
in fatty acids renders them particularly subjectible to oxidative
modification and thereby enhances their atherogenicity. |
|
|
|
|
Therapeutic Aim #6: Prevention of Damage from Oxygen Free Radicals Oxygen free radicals are promoters of atherogenesis. They
lead to structural impairment and to oxidative modification of lipoproteins
as well as other metabolic constituents.²³ Antioxidant nutrients such as
ascorbate, tocopherol (vitamin E) and beta carotene (provitamin A) can
protect against oxidative damage and against oxidative modification of
lipoproteins. Elevated plasma concentrations of these nutrients have been
shown to be associated with a decreased risk of CVD. 23,24
Nutritional supplements with antioxidative properties, including coenzyme Q
10 and selenium, contribute to maintaining optimum cardiovascular health. |
|
|
|
|
Therapeutic Aim #7: Optimum Cellular Function Optimum function of endothelial cells, myocardial cells,
smooth muscle cells, macrophages and other cell system critically determine optimum
cardiovascular health. Optimum metabolic function of these cells depends on
the availability of essential cofactors for a multitude of biochemical
reactions. Of particular importance are pantothenate, a cofactor for acetyl
coenzyme A, carnitine for fatty acid transport, the B vitamins for metabolic
energy transfer, ascorbate for enzymatic hydroxylations, and coenzyme Q10 in
the respiration chain. Optimum availability of these and other essential
nutrients, including certain minerals, not only helps protect the vascular
system but also improves cardiac function.24 The reduction of the
risk for CVD is, of course, also dependent on other factors, such as
exercise, cessation of smoking, and a prudent diet. |
|
|
|
|
Conclusion Effective reduction of the risk for CVD is a primary goal of the health care system in any industrialized country. In this paper I have presented new therapeutic approaches for this disease. Several of my earlier discoveries turned out to be of particular importance for these recommendations: The prominent role of lipoprotein(a) in human atherosclerotic lesions urged for new therapeutic approaches; the isolation of lipoprotein(a) particles from the vascular wall implied the reversibility of human atherosclerosis; the isolation techniques of lipoprotein(a) via lysine suggested the therapeutic use of this amino acid to induce this reversal. The report of the binding of lipoprotein(a) to proline¹³ suggested the therapeutic use of this amino acid in an analogous way. Most importantly my earlier discovery that lipoprotein(a) is primarily found in species which had lost the ability to synthesize ascorbate triggered a series of publications which may significantly improve our understanding of human CVD.¹’²’²º’²6 Ascorbate and several other nutritional supplements are of particular value including niacin, L-proline and L-lysine as well as natural antioxidants. The therapeutic use of these nutrients may pave the was towards a new therapeutic goal: the pharmaceutical, noninvasive reversal of existing CVD with nutritional supplements. |
|
|