![]()
| in association with cynthia a. glass, m.d.
|
L-Ascorbic Acid is an ingredient in the following SkinCeuticals Products |
|
ABSTRACT Recent research at Duke University demonstrates how topical vitamin C (L-ascorbic acid) benefits skin. First, the Duke studies show how to get large amounts of vitamin C into skin: the vitamin C must be in the form of L-ascorbic acid at low pH. Once in the skin, additional studies show that L-ascorbic acid has the following effects: it stimulates collagen synthesis, provides photoprotection, stays in skin for up to 72 hours, and prevents UV immunosuppression, a reaction which occurs in more than 90% of skin cancer patients. For reasons that aren’t entirely clear, sunscreens don’t fully protect individuals against UV immunosuppression. Topical vitamin C (L-ascorbic acid) protects skin against and reduces harmful effects caused by sunlight in both the UVB and UVA bands (290-400 nm). Although topical vitamin C does not absorb light in this range, and hence, is not a sunscreen, vitamin C (L-ascorbic acid) exerts its effects by neutralizing reactive oxygen species, the highly-reactive molecules produced when sunlight interacts with cell membranes and other components of skin tissue. And, unlike sunscreens, once vitamin C gets into skin, it can’t be washed, rubbed, or perspired off. Research shows that topical vitamin C (L-ascorbic acid) is an excellent antioxidant for skin protection and should be a useful adjunct to (but not replacement for) sunscreens. Recent scientific findings also reveal that it is the long UVAI (340-400 nm) rays that cause photoaging, and no currently available sunscreen fully protects individuals from all long UVA radiation. L-ascorbic acid is the only form of vitamin C that can be used by the body. However, it is notoriously difficult to stabilize and tends to break down rapidly, attributes that have prevented it from being used in cosmetic preparations. In order to solve the stability problem, many companies use derivatives of ascorbic acid, such as ascorbyl palmitate or magnesium ascorbyl phosphate. Before the body can use such derivatives, they must first get into skin and then be converted to L-ascorbic acid, a process that researchers would expect to be largely inefficient. The L-ascorbic acid formulation used in the Duke University research studies was stable in a laboratory setting. However, it lacked the long-term stability necessary for cosmetic use. Just recently, in an important cosmetic breakthrough, a stabilized form of L-ascorbic acid at low pH has been perfected for cosmetic use, and it is available now in some skin care products. This review first summarizes the science supporting topical vitamin C (L-ascorbic acid) skin care products. Then, it examines how sunlight and other environmental factors affect skin and L-ascorbic levels in skin. Finally, it highlights the differences between L-ascorbic acid products and those containing derivatives of vitamin C, and explains how to select vitamin C skin care products that work. THE SCIENCE The Physiology of Vitamin C The body does
not synthesize Vitamin C (L-ascorbic acid). It must be provided in
the diet. Body stores are limited by control mechanisms which allow
a maximum of 1200 mg. to be absorbed daily. The half-life of vitamin
C is 10-20 days, so that after three weeks, in the absence of
further ingestion, vitamin C is mostly depleted. The minimum daily
requirement for vitamin C is 200 mg. (Levine et al., Proc Natl Acad.
Sci, 1996). Vitamin C is a major antioxidant in the body. In
addition, it is important in collagen synthesis. L-Ascorbic Acid is an Inherently Unstable
Molecule
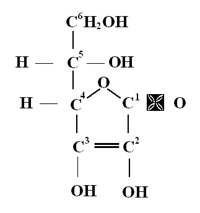
L-ascorbic acid is a moderately strong reducing agent. The number 2 and 3 carbons are double-bonded and contain an acid-ionizable hydrogen in water (pK = 4.2). These properties, which lead to instability in the L-ascorbic acid structure, are well known and have been burdensome to pharmacologists when attempting to formulate active, stable L-ascorbic acid solutions. At neutral or higher pH, as in most cosmetic formulations, L-ascorbic acid becomes the notoriously unstable ascorbate anion. For these reasons, among others, scientists have had difficulty formulating stable solutions of L-ascorbic acid that would be beneficial for cosmetic, dermatologic or opthalmic needs. Nevertheless, because or the many beneficial effects attributed to L-ascorbic acid, numerous attempts have been made to overcome these difficulties. A Stable, Topical Formulation of L-Ascorbic Acid Delivers More Vitamin C into Skin Than is Possible by Diet. After more
than a decade of research, scientists at Duke University have
developed a stable, aqueous formulation of L-ascorbic acid (vitamin
C), at acid pH-level (Darr and Pinnell, US patent 5,140,043, 1992).
They have proven how to get large amounts of vitamin C into the
skin, in levels that cannot be achieved by diet and are
pharmacological levels (Darr and Pinnell, US patent 5,140,043,
1992). Vitamin C is an Antioxidant Vitamin C (L-ascorbic acid) is a powerful antioxidant. Antioxidants protect skin by neutralizing reactive oxygen species generated when skin is exposed to sunlight, and which otherwise would destroy skin and its components (Shindo et al., J Invest Derm, 1994). Antioxidants work by neutralizing a series of oxygen molecular species, known as oxygen free-radicals, which damage and destroy skin. Reactive oxygen species are stimulated by ultraviolet light and also occur naturally during normal metabolism in the oxygen atmosphere in which we live. L-ascorbic acid neutralizes reactive oxygen species including superoxide anion, singlet oxygen and hydroxyl radical (Halliwell and Gutteridge, Arch of Biochem & Biophys, 1990). Topical Vitamin C Stimulates Collagen Growth Vitamin C stimulates collagen synthesis. In fact, it is the only antioxidant that has been proven to increase collagen synthesis. Collagen is the major structural protein of skin, and comprises 70% of its dry weight. The continued synthesis of collagen is essential to maintain healthy skin. Studies show that collagen decreases with intrinsic aging and that photoaging accelerates this process. In human skin fibroblasts in culture, vitamin C (L-ascorbic acid) stimulates collagen synthesis without affecting other protein synthesis (Freiberger, H. et al., J Invest Derm, 1980). L-ascorbic acid is a cofactor for two enzymes important in collagen synthesis: (1) prolyl hydroxylase and (2) lysyl hydroxylase. Prolyl hydroxylase is essential for producing a stable collagen molecule. Lysyl hydroxylase is necessary for cross-linking one collagen molecule to another collagen molecule; cross-linking is required for tissue strength (Colven and Pinnell, Clinics in Derm, 1996). L-ascorbic acid also serves as a transcription signal, relaying a critical message to collagen genes, telling these genes to synthesize collagen (Tajima and Pinnell, J Derm. Sci., 1996). Topical Vitamin C Protects Against and Reduces Harmful Effects of Sun in Skin Even the best sunblock on the market today does not protect fully in the UVA (320-400 nm) range. Antioxidants are, and presumably will continue to be, effective adjuncts to other skin protective products. New studies show that topical vitamin C (L-ascorbic acid) is an excellent antioxidant for UVA and UVB protection, making it a useful adjunct to (but not replacement for) sunscreens (Colven and Pinnell, 1996). Because topical vitamin C does not absorb light in the UVB/UVA range, it is not a sunscreen. Topical vitamin C works in two ways: it both protects skin against and reduces harmful effects caused by sunlight. It is equally effective in both the UVB (290-320 nm) and UVA bands (320-400 nm) (Darr et al, Br J Derm, 1992). Topical vitamin C (L-ascorbic acid) exerts its effects by neutralizing oxygen-free radicals produced when sunlight interacts with cell membranes and other components of skin tissue. Antioxidants Use A Different Mechanism To Protect Skin L-Ascorbic Acid Prevents UV Mutations Mutated cells cause skin cancer. L-ascorbic acid prevents UV mutations in skin cells. Therefore, some scientists believe topical vitamin C may prevent UV mutations that cause skin cancer. UVA generates reactive oxygen species that change DNA by breaking strands and mutating cells. There are only four “letters” in the language of DNA: G, C, A, and T, standing for the molecules guanine, cytosine, adenine and thymine. Repeated millions of times in varying combinations of pairs of C-G and A-T, they spell out control over all the inheritable characteristics of an organism. UVA light changes guanine into 8-oxoguanine, which may create a DNA mismatch; guanine, when in the 8-oxoguanine state can pair with adenine, rather than cytosine, creating a mutation. The more mutations, the more likely one is to develop skin cancer. It is estimated that cells in the human body get 10,000 of these insults a day, and are able to tolerate and repair the damage. However, when the cells in the human body are subject to more of these insults in one day (e.g., due to increased sun exposure unprotected from UVA insults), they cannot repair themselves fast enough. L-ascorbic acid prevents these UV mutations in skin cells (Stewart et al., J Invest Derm, 1996). Topical Vitamin C Prevents UV Immunosuppression Topical vitamin C prevents UV immunosuppression. (Nakamura, T., et al., J Invest Dermatol, 1997). This phenomenon, in which the activity of the immune system is stifled following exposure to sunlight, occurs in approximately one-third of individuals, on average. However, it is found in over 90 percent of those who get skin cancers, both melanoma and non-melanoma skin cancers (Granstein, R., Arch Dermatol, 1995; Streilein, W., in Gilchrest, B., Photoprotection, 1995). When skin is immunosuppressed, it is paralyzed in its ability to respond to sensitizers, such as poison ivy. For reasons that are not clear, sunscreens only partially prevent UV immunosuppression. Studies show that topical vitamin C prevents UV immunosuppression, specifically the loss of contact hypersensitivity in animals exposed to UV radiation and UVB-induced tolerance (Nakamura et al., J Invest Dermatol, 1997). However, it is premature to infer that topical vitamin C protects against skin cancer, because such studies have not been done yet. Topical Vitamin C is an Anti-Inflammatory Skin inflammation, including that caused by inflammatory dermatoses, phototrauma and carbon dioxide laser resurfacing is mediated by reactive oxygen species. Vitamin C, an antioxidant normally found in human skin, is depleted rapidly when skin is inflamed. Topical vitamin C has been reported to alleviate ultraviolet radiation-induced erythema on porcine skin (Darr et al., Br J Derm, 1992) and laser-induced postoperative erythema in human skin (Alster and West, Dermatolog Surg, 1998). Topical Vitamin C Controls Inflammation and Promotes Healing Topical vitamin C is capable of controlling the inflammatory response associated with ultraviolet light (sunburn). Topical vitamin C is protective even when it is applied after sun exposure (Darr et al., Br J Derm, 1992). Topical vitamin C also is helpful in speeding the healing process. It is often recommended as a pre- and post-operative regimen for laser resurfacing patients (Alster and West, 1998). Dermatologic surgeons recommend using it as long as possible prior to laser resurfacing and beginning again as early as fourteen days following surgery. Topical vitamin C also has been used successfully to treat rosacea patients, especially those tough patients who do not respond to other therapies (Bergfeld and Pinnell, Dialogues in Dermatology, AAD, 1996). Topical vitamin C serum (10% L-ascorbic acid) has been shown to decrease the degree and duration of CO2 laser-induced postoperative erythema, presumably because of its anti-inflammatory effect (Alster and West, 1998). Topical vitamin C serum (10% L-ascorbic acid) also has been used successfully to improve the appearance of striae alba or mature stretch marks (Ash et al., Dermatol Surg, 1998). Topical Vitamin C has a Reservoir Effect Topical vitamin C becomes an inherent part of the skin. It cannot be washed or rubbed off. Testing shows that it is fully protective for as long as three days after application (Darr et al., Br J Derm, 1992). This is known as a reservoir effect. HOW ULTRAVIOLET RADIATION AND OTHER ENVIRONMENTAL INSULTS AFFECT SKIN Skin is the Body’s First Defense Against Environmental Insults Human skin is constantly assaulted by environmental insults, including pollutants, pesticides, herbicides, heat, cold, and most prominently, smoking and sunlight. All of these insults generate reactive oxygen species, which include but are not limited to, oxygen-free radicals. Reactive oxygen species are a limited, but continually growing, family of small, oxygen-based molecules that either contain an unpaired electron or are capable of forming one. It is the process of oxidation that can destroy body tissues. This is the same process that causes cars to rust, and rubber tires to crack. Skin is the Body’s Largest Organ Not only is skin the body’s first line of defense against environmental insults, it also is the body’s largest organ. An average size person’s skin weighs six to seven pounds, and, if stretched out, would cover an area of twenty square feet. Human skin consists of two layers: (1) the epidermis, an outer, protective layer, which is about 0.1 mm thick, and (2) the dermis, an inner, living layer, which is about 1 to 4 mm thick (or 10 to 40 times thicker than the epidermis). Each layer has an important, yet specific, role in keeping skin healthy. The Epidermis The epidermis is a paper thin, protective outer coating which contains several layers of skin cells at varying stages of life. New cells form at the base of the epidermis and slowly move upward, losing moisture and flattening out as they go. By the time these cells reach the skin’s surface, they are dead and flat, and are sloughed off to make way for replacement cells. In addition to performing this important cell renewal function, the epidermis serves as an environmental barrier, preserving valuable moisture and protecting the body’s inner cells and organs from germs and other harmful invaders. The Dermis Sometimes
referred to as the “true skin,” the dermis or internal layer
accounts for 90% of skin mass. It provides structural and
nutritional support to the epidermis. The dermis contains many of
the major components in the complex functioning of healthy skin.
Consisting of dense, irregular connective tissue, the dermis also
contains small blood vessels, sweat glands, sebaceous (oil) glands,
fibroblasts, and nerves. It also contains elastin, which gives the
skin flexibility and durability. Finally, it contains collagen, a
protein that contributes to the skin’s firmness and can be
considered the structural steel of skin. Ultraviolet Radiation and The Ultraviolet Spectrum Exposure to ultraviolet radiation (UVR) is a well-documented health hazard. If it were not for a multi-layered defensive system, humans would die in the oxygen-rich environment in which they live. The ultraviolet spectrum is divided into the following key regions, which are measured in nanometers (nm), with each nanometer being one billionth (10-9) of a meter: UVC (270 - 290 nm), UVB (290 - 320 nm), UVAII (320 - 340 nm), and UVAI (340 - 400 nm). The ozone layer protects humans from damage against UVC rays, but not UVB and UVA rays. Reactive oxygen species, including oxygen free radicals, are generated by exposure of the skin to UV radiation. UVB rays are known to cause burning. UVA rays are now known to cause photoaging (Lavker et al., Photochem and Photobio, 1995; Lowe et al., J Invest Dermatol, 1995). UVB is heaviest during the hours of 10:00 a.m. and 3:00 p.m. and also during the summer. UVA is much more constant throughout the day and also throughout the year. UVA also can penetrate glass, including that of car, office and home windows; in contrast, UVB is blocked by glass. Approximately two-thirds of the UVA spectrum is UVAI, or long UVA. Compared to UVB, there is thirty times more UVA in the ultraviolet spectrum. What Happens When Sun Shines on Skin When sun shines on skin, the epidermis absorbs the short (290-320 nm) UVB rays. These generate oxygen-free radicals that can destroy and mutate cells and even cause skin cancer. The longer (320-420 nm) UVA (aging) rays go deep into the skin’s dermis, and even through skin. These rays penetrate thirty to forty times deeper than UVB rays, and also generate oxygen-free radicals. Oxygen-free radicals are like indiscriminate bombs, destroying and/or mutating anything in their way, including collagen, elastin, proteoglycan, cells and even DNA. Ultraviolet Radiation Destroys L-Ascorbic Acid in Skin When the skin is exposed to ultraviolet light, measurements show that two-thirds of the L-ascorbic acid in skin is destroyed. Ultraviolet light generates reactive oxygen species that may damage skin constituents including collagen, elastin, proteoglycan, as well as cell membranes and nuclear constituents. In time, it is believed that these changes may result in a breakdown in connective tissue. Visible signs of this destruction encompass intrinsic aging and photoaging changes – including wrinkles, solar lentigines (brown spots), actinic keratoses – and possibly even skin cancers. Scientists believe that L-ascorbic acid’s role as an antioxidant is essential in protecting the skin from the oxidative damage produced by ultraviolet light exposure, as well as the associated inflammatory reaction. UVA Radiation May Cause Photoaging and Skin Cancer Just recently,
a study co-authored by Duke University biophysicist, John D. Simon,
Ph.D., shows that UVA rays, a form of sunlight not blocked by most
products, may cause photoaging and skin cancer (Hanson and Simon,
Proc Natl Acad Sci USA, 1998). UVA Radiation May Play a Role in Melanoma Formulation A recent study
has detected a correlation between the use of sunlamps or sunbeds
and the development of melanoma, especially in younger individuals
(Autier et al., Int. J. Cancer, 1994). In addition, PUVA
(ultraviolet A radiation plus oral methoxsalen) therapy is known to
increase the incidence of melanoma (Stern et al., N. Eng. J. Med.,
1997). UVA radiation also is known to cause DNA mutations in cell
culture (Nishigori et al., J Invest Dermatol, 1996) and melanoma in
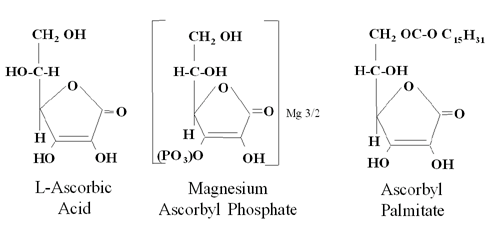
fish (Setlow et al. Proc Natl Acad Sci USA, 1993). DERIVATIVES OF TOPICAL VITAMIN C Not All Preparations of Topical Vitamin C are Effective Wanting to offer so-called “vitamin C” products, many companies have used derivatives of vitamin C, including ascorbyl palmitate and magnesium ascorbyl phosphate. Derivatives are easier to stabilize, but they are not L-ascorbic acid, which is the only form of vitamin C which the body can use. Figure 2 compares the L-ascorbic acid molecule with two common derivatives. Note the differences in structure between the simple, sugar-like L-ascorbic acid molecule and the more complex, but stable derivatives.
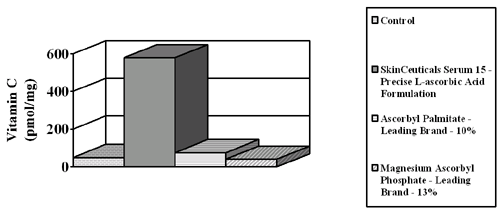
Recent studies indicate that vitamin C derivatives (e.g., ascorbyl palmitate, magnesium ascorbyl phosphate) which commonly are found in many skin care products do not perform in the same way as topical L-ascorbic acid. Studies to date show that derivatives are either not absorbed or not converted to L-ascorbic acid in high enough concentrations to have an effect (Kameyama et al., J Am Acad Dermatol, 1996; Murad et al., ASPRS, 1997). Research shows that percutaneous absorption of magnesium ascorbyl phosphorous is limited (Kameyama et al., J Am Acad Dermatol, 1996). The percutaneous absorption of ascorbyl palmitate has not been reported, but might be expected to prefer a cream vehicle to skin and thus remain outside, or on top of skin. Biologic activity of L-ascorbic acid and ascorbyl-6 palmitate also has been measured in human skin fibroblast culture at dosages need to stimulate collagen synthesis. Research showed that ascorbyl palmitate killed human skin fibroblasts at physiologic concentrations (100FM) by an unknown mechanism (Murad et al., 1997; Perricone, J Geriatr Dermatol, 1997) and was ineffective when compared to L-ascorbic acid against UV photoaging in mice (Bissett et al., Photodermatol Photoimmunol Photomed.,1990). PERCUTANEOUS ABSORPTION OF L-ASCORBIC ACID VERSUS DERIVATIVES L-ascorbic acid is an inherently unstable molecule, which is what makes it such a good antioxidant (Darr et al., 1996). To overcome the instability problem, many formulators use derivatives of vitamin C to provide stable cosmetic formulations. New scientific evidence shows decisively that derivatives don’t increase skin levels of vitamin C. They may get in, but they are not converted. In contrast, L-ascorbic acid gets into skin. The key is to get a high enough concentration of L-ascorbic acid into skin so that it can have an effect, and still preserve its stability. Study 1 Percutaneous Absorption Study Measuring Vitamin C Skin Levels After Application
Recent studies
indicate that vitamin C derivatives (e.g., Ascorbyl palmitate,
magnesium ascorbyl phosphate) that are commonly found in many skin
care products, do not perform in the same way as topical L-ascorbic
acid. Studies to date show that derivatives either are not absorbed
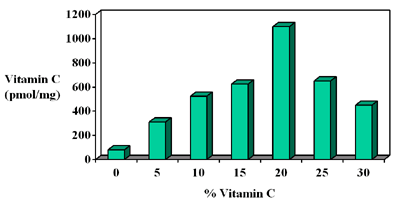
or not converted to Study 2 Vitamin C Dose Response New studies show that a 20% concentration of L-ascorbic acid gets the maximum amount of vitamin C into skin. These levels cannot be achieved by diet and are pharmacological levels.
Our lifestyles generate thousands of free radicals every day, which deplete levels of vitamin C in the skin. By applying a precise formula of 20% vitamin C, this assures that the skin has the maximum amount of vitamin C at all times to help fight free radical attack. EVALUATING TOPICAL VITAMIN C PRODUCTS Topical
vitamin C is the latest addition to the skin care arsenal. Because
of the scientific studies showing how it affects skin, many other
copycat products have emerged, undoubtedly confusing the consumer.
But all vitamin C products are not created equally. So, how does one
evaluate a topical vitamin C product? 1. Is the vitamin C in the form of L-ascorbic acid, the only true form of vitamin C, and the only form the body can use? 2. If yes, is the L-ascorbic acid at a low pH so that it penetrates skin and a high enough concentration to have an effect? 3. Is the preparation stable? It took Dr. Pinnell and the other Duke scientists over a decade to find a way to stabilize L-ascorbic acid, and the initial preparation was stable only in a laboratory environment. Since then, a stabilized form of L-ascorbic acid at low pH for cosmetic use has been perfected which incorporates the Duke-patented technology, and this formulation is available only in SkinCeutical’s topical vitamin-C products. Therefore, if the vitamin C is not in the form of L-ascorbic acid, another set of questions must be asked: 1. What is the scientific evidence that the product gets into skin? 2. If it gets into skin, what is the evidence that the product is chemically converted to L-ascorbic acid? 3. If it is converted to L-ascorbic acid, what is the resultant concentration of L-ascorbic acid? What Makes a Topical Vitamin C Product Effective? To be effective, a topical vitamin-C preparation should contain a high concentration of L-ascorbic acid in a stable, aqueous, acidic pH formulation. Why are these factors important? High Concentration. Ten percent concentration appears to be necessary for an optimal biological effect. Many cosmetic products contain ascorbic acid or ascorbic acid derivatives, but most contain concentrations less than 3%. L-Ascorbic Acid. The only form of ascorbic acid that can be used by the body is L-ascorbic acid. Ascorbic acid is most commonly supplied as an equal mixture of D-ascorbic acid and L-ascorbic acid. These forms of ascorbic acid are stereoisomers, but the body uses only L-ascorbic acid. A concentration of 20% of the commonly-supplied ascorbic acid would be necessary for an equivalent biological effect. Stability. Ascorbic acid is notoriously difficult to stabilize, which has precluded its use as a general cosmetic ingredient. The technology for allowing ascorbic acid to enter skin is unusual. Duke University was awarded a patent precisely because it succeeded in stabilizing L-ascorbic acid and getting it into skin, which is something cosmetic companies had tried, but failed to do, for decades. What are the Problems with Ascorbic Acid Derivatives, Esters and Analogs? In an attempt to solve the problem of stability of ascorbic acid in cosmetics, manufacturers have substituted derivatives, esters and analogs of ascorbic acid. These include ascorbic acid sulphate, ascorbic acid magnesium phosphate, ascorbyl stearate, ascorbyl palmitate, ascorbyl dipalmitate, and magnesium ascorbyl phosphate. In order for any of these compounds to work in skin, they must first get into skin and then be chemically converted by cells in the skin into L-ascorbic acid. Getting
Compounds Into Skin. It is very hard to get chemicals into
the skin. Otherwise, we would use topical formulations to deliver
all of our drugs. Fortunately, the skin is very efficient at
protecting us from foreign substances. There is no direct evidence
that ascorbic acid derivatives enter the skin in appreciable
amounts. Moreover, it would be predicted that salts, such as
ascorbic acid sulphate and ascorbic acid magnesium phosphate, would
not enter because of their charged nature. Esters of ascorbic acid,
such as ascorbyl stearate or ascorbyl palmitate, might be expected
to chemically prefer the environment of a cream to the environment
of the skin and therefore not substantially enter
skin. How to Find a True Topical Vitamin C Product Look for products that contain stable L-ascorbic acid at low pH and high concentration. It is easy to determine whether a product is color stable, just by looking at it over time; determining whether the L-ascorbic acid is stable is more difficult, but possible, and typically requires laboratory analysis. Color stability is a good substitute indicator. Finally, remember that just because the label says “vitamin C” doesn’t mean the product contains L-ascorbic acid! SUMMARY Recent research indicates that topical vitamin C (L-ascorbic acid) is an excellent antioxidant for skin protection and should be a useful adjunct to sunscreens. Recent scientific findings also reveal that it is the long UVAI (340-400 nm) rays which cause photoaging, and no currently available sunscreen fully protects in this range. Topical vitamin C both protects against and reduces harmful effects in skin caused by sunlight in both the UVB and UVA bands (290-400 nm). Although topical vitamin C does not absorb light in this range, and hence, is not a sunscreen, vitamin C (L-ascorbic acid) exerts its effects by neutralizing oxygen-free radicals, the highly-reactive “bombs” produced when sunlight interacts with cell membranes and other components of skin tissue. Research studies show that a topical vitamin C preparation at acid pH has useful properties. It can be targeted directly into skin and provide pharmacological levels of protection. It becomes an inherent part of the skin, and is unaffected by bathing, exercise, clothing, or makeup. It is believed that it will be useful for treating sunburn and other inflammatory conditions, including acne, rosacea and erythema. It is safe and should be attractive for use on adults and children. Topical vitamin C is a useful adjunct to sunscreens. Based upon the most recent research, two key steps are recommended to repair and protect your skin from photoaging and other oxidative damage: 1. Protect your skin by using a product containing a high concentration of L-ascorbic acid at a low pH so that it penetrates skin. 2. Follow with a broad spectrum moisturizer which provides UVB, UVAII and UVAI protection and has an SPF 15 or higher daily. For the strongest defense against photoaging, adopt a skin care regimen that includes products which meet the above criteria. 1. Pinnell,
S.R. and Madey, D.L. Topical Vitamin C in Skin Care. Aesthetics
Surgery Journal. November/December 1998 (in press). - ### - L-Ascorbic Acid Serum 15
|
|
Cffectives
Vitamin C New from Obagi |