
Before venturing into a summary of the ways in which gems are formed, it would be good background to review where those processes are occurring. A crude geological model of our Earth can be made by using an apple. The Earth's crust or top layer (ranging from 3 - 25 miles deep, only 1% of the Earth's volume) is represented by the thin skin, its mantle accounting for over 80% of the volume is represented by the flesh (over 1800 miles thick), and the core is represented by, what else?, the core.

The core has a solid inner, and liquid outer, portion, and is the least known and studied part of the Earth. Most of the mantle consists of melted rock (magma) and giant segments of the Earth's crust along with the solid upper mantle, called "tectonic plates", float on this fluid rock and move slowly over it. The movement normally occurs at about the same rate your fingernails grow, but can be substantially faster when one plate suddenly slips in relation to another. The various ways in which these plates encounter each other, are responsible for many of the momentous events that shape our Earth, such as volcanism, earthquakes, and periods of mountain building.
Almost all gems of mineral origin form in the Earth's crust, with the notable exceptions of peridot and diamond, which form in the mantle, and all of them are mined in or on the Earth's crust. This gemiferous crust is made up of three types of rocks: igneous, sedimentary and metamorphic, which differ in their origin and characteristics. Igneous rocks are those which solidify from a molten state, sedimentary rocks form due to consolidation of layered sediments , evaporates, or precipitates, and metamorphic rocks result when great temperature and pressure change the crystal structure of either igneous, sedimentary, or other metamorphic rocks. (Recall from Lesson One, that minerals are composed of a single substance, like quartz, and rocks are made of more than one mineral, like granite, for example, which consists of quartz, mica and feldspar.)



**Check the web: This site, although meant for kids, is well done and worth seeing. It has animations of the conditions under which the three types of rocks form: http://www.fi.edu/fellows/fellow1/oct98/create/index.html
The rocks that we find on, and under, the Earth's surface are involved in an age-old, and continuing, recycling program of Nature called the "Rock Cycle".
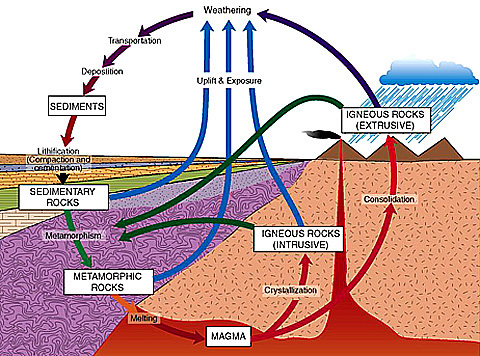
Let's start with the red area at the bottom of the diagram: magma, molten rock. When magma rises through cracks and cools slowly underground, it forms igneous rocks composed of minerals with fairly large crystal sizes, these are known as intrusive igneous rocks. When the magma erupts onto the surface, as through a volcano, it is termed lava, and depending on the rate of cooling, the extrusive igneous rocks which form have medium to very small mineral crystals. (Review the concept of temperature and crystal size from Lesson Three, if necessary). Some lava cools so rapidly that it forms an amorphous material without a crystalline structure. Granite and basalt are examples of larger and smaller grained igneous rocks, respectively, and obsidian (volcanic glass) is amorphous.
[Granite: note coarse texture: Image courtesy of Dr. Barb Dutrow] Once the igneous rock is on the surface, the forces of erosion and weathering produce smaller particles which accumulate on the surface, and/or are moved by wind and water. As time proceeds, layers of these sediments build up (on land or under water). The pressure from upper layers causes compaction in the lower layers along with various chemical and physical changes (lithification), which lead to the creation of sedimentary rock.
Evaporation is an alternate factor which also produces sedimentary rocks, as when dripping mineral-laden waters leave behind stalactites or stalagmites. Likewise, surface or subterranean waters carrying dissolved minerals may evaporate or precipitate those minerals within the cracks in other rocks, or between rock layers. Sandstone and limestone are familiar sedimentary rocks formed by lithification. Opal and turquoise illustrate the evaporative mode of formation.
The presence of intrusive magma in a local region (contact metamorphism), or of tectonic plate interactions on a larger scale (regional metamorphism) puts the igneous and sedimentary rocks and minerals under heat and/or pressure which may cause changes in their chemistry and crystal structure. The result is the creation of metamorphic rocks. Thus is limestone turned into marble, sandstone into quartzite, and serpentine into nephrite jade.
As with most cycles in Nature there are sub-cycles and cross interactions. So, for example, sedimentary rocks which are subducted through tectonic action may melt and form magma which produces igneous rocks. Or metamorphic rocks, which have been uplifted and exposed at the surface, will erode to form sedimentary deposits.
A specific, and unlikely, combination of five factors: temperature, pressure, space, chemical elements, and time, are required for the formation of each kind of gem. This is why gems are, in general, rare-->but some are rarer than others. Silicon and oxygen are the two most abundant elements of the Earth's crust, and the conditions for the formation of quartz (SiO2) are relatively common, so it is understandable that quartz is found widely. Axinite on the other hand, which is also a silicate gem, requires (in addition to silicon and oxygen) calcium, iron, magnesium, boron and aluminum for its formation, and is much rarer.
Oxygen: 46.60%
Silicon: 27.72%
Aluminum: 8.13%
Iron: 5.00%
Calcium: 3.63%
Magnesium: 2.09%
Boron: 0.0010%
Beryllium: 0.00026%
Gems, in Nature form: 1) from solutions by precipitation, 2) from melts by crystallization, or 3) from vapors by condensation. (You may recall from Lesson Eight that when Man synthesizes gems, these are also the three possible modes of production.)
Both near-surface, cooler waters, and warmer waters from lower depths in the Earth, can dissolve certain minerals from rocks or sediments, and carry, mix, and concentrate them until conditions change, ultimately precipitating them as solids (crystals or amorphous materials).
Near surface environments: Near surface waters, like rainwater, move down or up, through soil or rock, as the local cycles of precipitation and evaporation dictate. Such water has carbon dioxide from the air dissolved in it, which creates a weak acid solution (carbonic acid) in which many minerals are soluble. If the environment contains sandy soils or sandstone rock, then silica will be dissolved, and certain silicate gems such as aggregate quartzes, like agates, or amorphous opals may form as the water evaporates.
Commonly, layered or banded patterns are seen in the agates indicating cycles of formation from waters of slightly different chemistries. The botryoidal habit is also frequently seen in gems formed under near surface conditions. Likewise ocean water or other brines can evaporate as climate changes leaving behind dissolved minerals, like halite (the mineral name for sodium chloride, table salt). Other waters containing sulfur may evaporate, and leave behind sulfate minerals like gypsum.
If the rocks or soils contain aluminum and copper in addition to silica, then copper containing minerals like azurite, malachite and turquoise may form.
[Near surface silicate gems: agate cabochon showing layered structure, botryoidal carnelian, precious opal in rock seam, common opal nodule] [Amethyst stalactite: note layered structure of both aggregate and single crystals] [Minerals from briny evaporates: "cranberry halite" from Nevada, green halite from Australia (color is due to pigments from crustaceans and microorganisms that lived in the salty water), gypsum "roses": Images courtesy of Las Vegas Jewelry and Mineral] [Turquoise bearing rock, from Nevada: Image courtesy of Las Vegas Jewelry and Mineral, rare occurence of single turquoise crystals from Virginia 50x, malachite, turquoise and chrysocolla veins in an Arizona rock: Image courtesy of Dr. Barb Dutrow, slice of a malachite and chrysocolla stalactite: Image courtesy of www.barlowsrocks.com, azurite crystals from Utah] Think about where you imagine miners finding agates, opals, and copper minerals--> you probably already know that the best deposits occur in rocky, sandy areas with an arid or semiarid climate. (Most of the world's precious opal, for example, comes from the Australian desert, and the Western USA and Mexico, are well known sites of turquoise and agate deposits).
[Map of some of the major turquoise mines in the Southwest (photo taken at the Las Vegas Natural History Museum)] Petrifaction: Sometimes the hard, organic remains of plants such as wood or cones, or the bones or shells of animals are buried in lava or sediments before they can decay. Such burial restricts oxygen supply, and decomposition processes slow to a snail's pace. Silica laden waters can, ever so slowly, fill and replace any cavities or structures that are present with agate or opal, preserving a replica of the original form in solid rock. Many fossils are the result of this process, known as petrifaction.
[Examples of petrifaction: slice of fossil palmwood: Image courtesy of www.barlowsrocks.com, cabochon of fossil palmwood from Texas, fragments of fossilized dinosaur eggshell, pendant with red spinel, fire agate and fossilized dinosaur eggshell, fossil wood slices from Oregon: Image courtesy of Las Vegas Jewelry and Mineral, opalized clam fossil (opal solution filled the cavity of the clam shell and solidified before the shell decayed. Remnants of the fossil shell were then cut and polished away, revealing a perfect "cast" of the original shape] Deeper Environments: Waters from deeper in the Earth are often heated from contact with hot rock, and are sometimes highly acidic or alkaline, making an even better solvent for more types of minerals. Environments where water of this type is found are termed "hydrothermal". Usually, rates of cooling and/or evaporation are slower than in near surface environments giving time for single, larger crystals to form. Many of the world's highest quality mineral specimens and metal ores have come from such hydrothermal sources. Emeralds, rock crystal quartz, amethyst, and fluorite are gems commonly formed when hydrothermal fluids solidify (as veins or crystals) in the cracks or pockets within rocks, or between rock layers.
[Hydrothermal amethyst crystals from Mexico: Image courtesy of www.irocks.com, gold veins in quartz: Image courtesy of California Geological Survey, native copper veins in Arizona rock: Image courtesy of Dr. Barb Dutrow, hydrothermal fluorite crystals, dendritic silver in quartz: Image courtesy of www.irocks.com, Natural hydrothermal emerald crystals in matrix: Image courtesy of www.yourgemologist.com] Geodes: Cavities dissolved into sedimentary rock, or gas pocket cavities in igneous rock, are prime sites where crystallization from hydrothermal solutions occur. The results, known as geodes, usually contain agate or quartz, and are one of the favorite finds of rock hounds.
[Small quartz geodes, huge amethyst "cathedrals": Images courtesy of Las Vegas Jewelry and Mineral, the outside and inside of a rare azurite geode from Arizona]]
As magma cools, various minerals form, depending on the temperature and pressure at a particular location and time. As each type of mineral forms it reduces concentration of, or removes, some of the elements required for its formation. Thus as the mix of elements present, and the physical conditions change, so do the minerals which form.
Intrusive: Gems usually form in intrusive igneous rocks where the slow rate of cooling favors larger crystals. Generally, though, we do not mine the original formation sites of these gem-containing rocks, but instead gather the weathered-out gems which have been released when these intrusive rock bodies are uplifted to the surface, or erosional processes reveal them. Corundum and topaz are examples of gems which form in intrusive rocks.
Extrusive: Extrusive igneous rocks would generally not be expected to hold large crystals. Occasionally, though, some large crystals will form deep underground, but before crystallization of other minerals is complete and a typically large grained intrusive rock is produced, the magma suddenly finds its way to the surface. Under these new conditions, the rest of the magma (carrying the large crystals from below) quickly solidifies to becomes fine grained rock. In such extrusive igneous rocks we find larger gem crystals in a matrix of finer grained rock. (See mantle gems, below). Corundum, moonstone, garnet and zircon are examples of gems that can be formed and brought to, or near, the surface in this way.



Gems formed in the mantle: Peridot crystals form in magma from the upper mantle (20 to 55 miles deep), and are brought to the surface by tectonic or volcanic activity where we find them in extrusive igneous rocks. Diamonds were formed many millions of years ago, deeper in the mantle (around 100 - 150 miles below the surface), at extreme temperatures and pressures. These diamond forming magmas would later erupt (still holding the diamonds) to form rocks called kimberlites and lamproites.
[Diagram courtesy of The International Gem Society and Don Clark, www.gemsociety.org] The scenario goes something like this: 1) magma, containing diamond crystals, suddenly and explosively finds a path to the surface. 2) As the lava (orange) rises, some of it cools and solidifies underground forming a carrot shaped formation of kimberlite rock, in which the diamond crystals are "frozen". 3) & 4) The volcanic cone has eroded away leaving diamonds at the surface, and underground in the kimberlite (or lamproite) "pipe" (gray).
Pegmatites: As magma, which contains dissolved minerals in water under pressure, begins to rise through cracks and cool down, crystallization begins. The magmatic water, along with the dissolved minerals which require lower temperatures for their crystallization, becomes more and more concentrated. In the end phases of crystallization of the magma, the water is expelled as vapor, and the highly concentrated magma remnants crystallize near the surface in a distinctive geologic formation known as a pegmatite. The magmas from which pegmatites form often contain high concentrations of rarer elements like beryllium and boron. Gems commonly found in pegmatites are emerald, topaz, tourmaline, rose quartz, chrysoberyl and spodumene, and they can be very large.
** Check the text: look up the chemical formulas of tourmaline and emerald and see which of them requires beryllium, and which boron. Check page 83 in Hall to see what rare element is responsible for the pink color in rose quartz.
[World class aquamarine crystals from N. Pakistan pegmatite: Image courtesy of www.irocks.com, pink tourmaline rough from a pegmatite formation in the Stewart Mine in California, emerald crystals: Image courtesy of Las Vegas Jewelry and Mineral, rose quartz from a Brazilian pegmatite mine: Image courtesy of www.irocks.com ] **Check the Web: One of the most famous pegmatite mines in the US is the Stewart Tourmaline Mine in Pala, California. This famous deposit, most noted for its bubble gum pink tourmaline, consists primarily of pegmatite formations of a type called dikes. Visit this link to take a virtual tour of the mine: http://www.mmmgems.com/stewart/minetr2.htm
Vapor/Condensation Formation
It might be a little difficult to imagine vapors condensing to form crystals, as it seems somewhat foreign to every day experience. And it's true that at normal atmospheric pressures, and common ambient temperatures, this doesn't happen very often. But there's one good example that we can all look to: frost which forms on our windowpanes or car windshields, is, in fact, precisely a situation of a vapor (water vapor) condensing to a solid crystal (ice). The next time you get a chance--> use your loupe to example that frost: beautiful! Given the extreme environments created by some geological events, such as an eruption of magma, conditions can be ideal for such condensation processes, and they are relatively common events.
Vugs: When magma (a fluid with dissolved liquids and gases) is suddenly released from the pressures containing it (as when it erupts or spreads into surface fissures), gases are freed and liquids quickly vaporize to gas, which creates gas-filled bubbles and pockets in the lava called "vugs". (We experience a similar phenomenon every time we open a carbonated beveridge).
Gems cans crystallize from these vapors which are trapped and concentrated inside the openings. Often they form singly, without attachment to the surrounding surface. When we see a doubly terminated crystal, or one that is perfectly formed with no attachment point (called a "floater"), often it has formed in just a such a gas pocket. One of the most famous deposits of these doubly terminated crystals is the rock crystal quartzes formed in Herkimer, NY, and known as "Herkimer Diamonds".
Other pockets which do not produce crystals from gases, may later be invaded by surface water, or hydrothermal fluids, and become filled or lined with small or large crystals forming geodes or other similar formations.
[Spessartite garnet "floater" crystal from Namibia, doubly terminated rock crystal quartz ("Herkimer Diamond") from New York, igneous vug lined with hydrothermally derived quartz crystals, vug from Germany, containing stalagtites covered with tiny quartz crystals: Image courtesy of www.irocks.com] Crystal growth from solutions or vapors can also exploit fortuitous openings as seen below. This ancient clam's death, and subsequent fossilization, created a space in the surrounding rock which later became home to the beautifully formed calcite crystals in this prize specimen.
[Fossil clam shell with calcite crystals, from Okeechobee County, Florida]
As mentioned previously, heat and pressure from contact or regional sources can cause one mineral to metamorphose into another. This frequently occurs with gem minerals. Marble and lapis lazuli are gem rocks formed metamorphically, and rubies, spinels and garnets are gem minerals are often crystallized within rocks that are undergoing metamorphic changes, due to heat and pressure.



** Check the text: See Lyman, page 38, for a picture of metamorphically created almandine garnets, and page 12 in the Hall book to for kyanite and staurolite in metamorphic schist
Thus far only those originally organic gems that have become fully mineralized through petrifaction, have been referred to in this lesson. Another lesson of equal length could be written about the precise mechanisms by which the various true organic gems come to be. Such coverage is beyond the scope of this course, however; so the following inadequate summary must suffice. (Review the definition of mineral vs organic gems from Lesson One, if necessary).
Organic gems are derived from either:
1) The secretory activities of organisms that were living (or recently dead) at the time of the harvest of the gem material.
Examples would include pearl, bone, horn, ivory, tortoiseshell and coral. Such secretions might be entirely mineral, as with the calcareous corals, for example, or entirely organic as with tortoise shell, or a combination of both mineral and organic components as with pearl, proteinaceous corals, and ivories.
[Warthog tusk (ivory), Victorian Ivory brooch, 1930's natural tortoise shell brooch, raw black coral branches (proteinaceous type): Image courtesy of Las Vegas Jewelry and Mineral, polished black coral branch pin with Tahitian pearl] 2) The secretions and structures of organisms long dead which have, over time, undergone geological and/or chemical changes from their original state.
Examples include amber, copal, jet, and bog oak. Chemical oxidation or reduction, compression, dehydration, or polymerization, have changed their original properties, but these materials still consist, at least partially, of organic molecules.
[Circa 1880 "bog oak" brooch (a semi-fossilized wood from ancient peat bogs, similar to, and used as a simulant for, jet), amber rough: "Burmite" very rare Burmese variety, noted for its cherry red color, typical Baltic amber]
Where Gems are Found and How they are Mined
An important distinction must be made between the place where a gem forms, and where it is mined or collected (these two, most often, are not the same). The places where we mine or collect gems are known as gem deposits, and these are classified as either primary or secondary.
A primary deposit is one in which the sought-after material is still held within the original site of its formation. These "lode" deposits are often located deep underground, and encased in solid rock (pegmatites, veins, pipes, etc.) They are, in general, likely to require substantial monetary outlay in personnel and equipment for recovery.
Although metal ores (does the famous Comstock Lode come to mind?), are frequently mined from primary deposits, it is rarer with gemstones. In certain locations, though, diamonds, and colored gemstones can be profitably mined from such sites. Techniques involve either tunneling deep into the Earth, or using open pit technology necessitating removal of massive amounts of "overburden" to get to the deeper gem bearing layer.
**Check the web: Get a moving panoramic view of the famous, "Lavender Pit" copper mine in Bisbee, Arizona at this site. This, now abandoned strip mine, named for Harrison Lavender, an executive with Phelps Dodge Company, encompasses 300 acres, is 950 feet deep, and yielded 351 million tons of ore during its active life: http://virtualguidebooks.com/Arizona/CactusCountry/Bisbee/LavenderPit.html
A consideration which is important in this type of gem mining is that the typical blasting and crushing done with metal ore materials can harm fragile gem crystals, so that much of the work must be done by slower and more labor intensive hand work. Sapphires in the US, have been "hard-rock" mined, off and on (depending on economic factors) in Montana, primarily at Yogo Gulch. The deposit there consists of sapphire cyrstals in a lamproite pegmatite dike. Although they are some of the highest quality blue sapphires in the world, lacking color zoning, and possessing an "out of the ground" cornflower blue color that requires no heating, the extreme prices necessary to repay their mining costs, limit their marketability.


** Check the web: Here's a site with pictures and text showing one of the newest of De Beers' South African diamond mines. It explains some of the steps involved in extracting and processing diamonds, at a recovery rate of approximately one carat of diamond per ton of ore. http://www.mining-technology.com/projects/de_beers/
Although a primary deposit may have been formed deep in the Earth, uplift, crust folding, or other geologic events can bring it to, or very near, the surface. All exposed surface features are subject to erosion and weathering, and this is true of gem deposits as well. The agents of erosion will then act to release the gems from their primary sites, and they collect in new secondary deposits. Secondary deposits are classed as either eluvial or alluvial depending on their relationship to the original source.
Eluvial: When the softer more easily weathering primary structures simply release the harder and tougher gem materials, and the gems can be found at the site of decomposition, the deposit is eluvial. The gems can then be located within the debris, and generally it will be a relatively inexpensive process to gather and remove them. Additionally, the host rocks, which may contain valuable primary deposits can usually be easily located for other types of mining.
Eluvial gem rough, although often large in size, tends to be internally fractured, and quite angular and irregular in shape, which can limit its potential as faceting material.
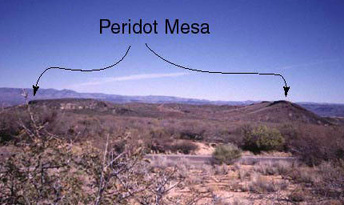
The world's largest peridot mine, located on the San Carlos Apache Reservation in Arizona, is an eluvial deposit where the peridot is weathering out of the volcanic basalt primary source.


Alluvial: More common, and in most cases, more desirable, are alluvial (also known as "placer") deposits. The gems in these have been transported from the original site of their release, usually by water, but also possibly by wind or ice. As most gems are both denser and harder than most rocks, they accumulate on the bottom along with gravel, sand and mud, in eddies and pools in streams, rivers and along coastlines. (They can also be found in sites that had flowing water in the past, but have long since dried up).
The abrasive and frictional forces that occur as the gems are moved downstream cause the weakest parts to break off, and the edges to become more smooth and rounded. Alluvial rough, though usually relatively small, is often of high clarity, and superior faceting quality. The longer the distance the rough has traveled, the smaller and more rounded it becomes. Alluvial diamonds are an exception to this rule, in that they are harder than the surrounding rocks, and unless fractured or cleaved retain their original structure and size.

By far, the greatest amount of economically profitable gem mining is done by exploiting secondary deposits. Techniques range from simple one person panning and screening operations, to large scale dredging and hydraulic washing/sorting by big companies.
Food for thought:
Question 1: Dealers who supply facetors with gem rough may sometimes tumble it first. Why?
Question 2: What type of terrain do you think is most likely to yield eluvial deposits? alluvial ones?



Within alluvial "gem gravels" several different types of gems may be found together, reflecting the various eroding primary sites within the local drainage area. Tracking back to the primary source of a particular gem ("Mother Lode") is usually very difficult, if not impossible.
Answers to the thought exercises for this lesson. (If you don't understand something in these answers it's time to email me and ask!)
1): Gem rough that comes from primary sources or eluvial sources may have internal fractures, partial cleavages, or ungainly shapes. Tumbling the rough simulates what happens as weathered-out gems travel down streambeds, breaking off weak areas and wearing away protrusions. The tumbled rough is more desirable to the facetor as it is cleaner, and better shaped for good recovery; so they will pay more for it.
2): Flat terrain, especially that which is in an area that is arid, is most likely to yield eluvial gems. It is not surprising, then, that we find eluvial peridot at a place named "Peridot Mesa" (mesa = table top). Alluvial gems however, are most likely to be found in the foot hills and valleys of mountain ranges. (Where did the '49ers look for gold nuggets?).
You have now completed the web lecture for the tenth lesson!
Go back the the course web site to: 1) complete and submit the homework assignment on the text readings and assigned web essays 2) take the non-graded practice quiz on this web lecture 3) post a comment to the discussion board for this lesson, and 4) when it is available, complete the graded quiz based on this web lecture.
Now Get Ready for the Final Exam!!