Enhancement is defined as any processing (other than fashioning) which improves the appearance or durability of a gem. Determining whether or not a gem is enhanced is part of the previously discussed aspects of gemological investigation:
1) Species: What is this gem?
2) Origin: Is it natural or synthetic?
3) Treatment: What enhancements, if any, has it received?
Just to think about: Does it matter if a gem is enhanced?
So what, if a gem is enhanced? If enhancement makes it prettier, or more durable, what's the big deal, and why do we even need to know? Here are some of the major issues to consider:
- Enhancement can alter the value of a gem up or down. If it makes an unuseable piece of gem material useable, or an ugly one pretty, then it has increased the value of that particular piece. On the other hand, the reality of the marketplace is that "absence of treatment" in itself, has value. (Why this is so has to do both with rarity, and with a certain philosophical value many accord to the unaltered products of "Mother Nature".)
Take two equally clean, natural origin rubies of exactly the same size, cut and color: one has the color it came out of the ground with, the other is a piece that originally was a less attractive color, but has been color enhanced by heating. In today's market the unenhanced stone would bring from 10 - 20% more per carat.
**In this case, the reasons for needing to know about a gem's treatment status are economic and philosophical.
- Although many enhancements produce permanent changes, others are unstable and fade, wear away, or alter with time and exposure to environmental factors. The man-made iridescent topazes that were discussed in the last lesson are good examples here. The coating is microscopically thin, and care must be taken to preserve it. On the other hand, the blue colors of irradiated and heated topazes (like Swiss and London blue), are permanent and stable, and these gems do not require any different level of care than naturally colored ones.
**In this case the reasons for needing to know about a gem's treatment state are practical.
The general feeling among gemologists and ethical gem merchants is that there is nothing wrong with any type of enhancement, as long as it is fully disclosed (including care instructions), and the gems are appropriately priced to reflect their treatment status. Unfortunately, there are many "ethically challenged" companies and individuals which seek to profit by doing neither of these.
FTC: The Federal Trade Commission regulates many basic aspects of marketing, advertising and commerce in general. For gems and jewelry, the pertinent regulations regard certain aspects of advertising and describing gems, as well as issues of gem weights and measurements.
AGTA: The American Gem Trade Association is an industry organization of carefully screened colored gemstone dealers who are based in the USA. Attaining membership in this organization involves a lengthy and rigorous vetting process meant to assure that only dealers who ascribe to the highest standards of ethical business practices can belong. This organization has had extreme influence, world-wide, primarily by developing and publicizing standards of ethical business practices for colored stone dealers, especially as regards disclosure of enhancements.
AGTA guidelines for gem advertising and marketing go well beyond the generalized and generic ones found in the FTC regulations. Because more and more countries are adopting the AGTA guidelines and their coding system, or patterning their own after it, we will be covering portions of it in this class.
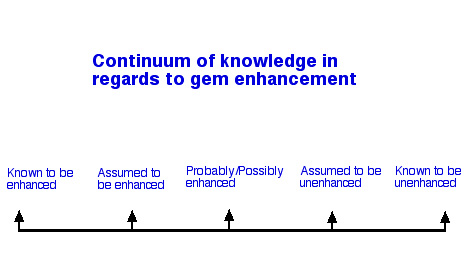
On the one end are gems that we know, 100% for sure, are enhanced. This might be because the treater has presented the material to us as enhanced, for example: the invoice says "heat treated sapphire" or "dyed chalcedony". It could also be because in examining the goods we find incontrovertible evidence of enhancement. For example: microscopic examination of a diamond reveals the characteristic tunnels made by lasers for purposes of clarity enhancement, or testing the surface of a gemstone bead with a swab dipped in acetone removes some of the blue dye from it.
On the opposite end are gems that we know, 100% for sure, are unenhanced. This category is pretty small actually, as unless you "dig it yourself", you are taking the word of someone else as to the gem's treatment status. (Even in this case, it is possible that the enhanced gem rough could have been "salted" into the natural source!)
Not all enhancements can be revealed by current testing methods, so in some cases a thorough (and costly) examination by a trained professional might only give the equivocal result of: "no evidence of enhancement found", which is not the precisely the same thing as "unenhanced". Many low temperature heating processes, and some forms of irradiation are literally undetectable with today's technology. They leave no tangible signs distinct from those which might have been the result of natural environmental factors.
Inbetween these extremes, lies our degree of knowledge of the treatment status of most gems: three major stopping points on the continuum might be labelled:
- Assumed to be enhanced
- Probably/Possibly enhanced
- Assumed not to be enhanced
Gems which are assumed to be enhanced are those species in which enhancement is the standard practice, or those which don't exist, to any extent, in Nature in the treated color or form . Examples would be blue topaz, and black onyx which are found in only tiny quantities in Nature, but produced in huge amounts by irradiation, and dyeing, respectively. This category would also include oiling of emeralds, a process used on more than 90% of emeralds in commerce.
Gems which are probably or possibly enhanced are those for which known treatments exist, but range from being commonly to occasionally used. Examples would be resin "stabilization" of turquoise, bleaching of pearls, dyeing of jade, and heating and/or irradiation of beryls , quartzes and tourmalines. It is in this situation that familiarity with the detectable signs of enhancement can be most useful. A major goal of this lesson is to acquaint you with some of the most important of these.
Gems which are assumed not to be enhanced include those for which no treatment has yet been discovered for the material (or at least none that is cost effective and non-destructive). This category is highly provisional as new enhancement processes may be developed or their safety or economics improved at any time. Examples of gem species which currently can be assumed to be unenhanced are: spinel, iolite, sunstone and most types of garnets.
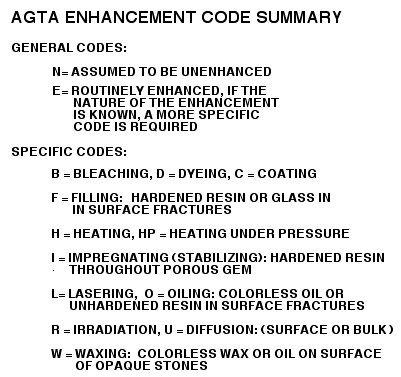
[2 general and 12 specific AGTA codes to be familiar with] History of Gem Enhancement:
The quest to make gems look better, last longer, or sell at a higher price is nothing new!:
- As far back as 2000 BCE the Minoans applied thinly beaten gold foil to the back of transparent stones to make them more reflective.
- Amongst the treasures buried with "King Tut", circa 1300 BCE, were heat treated carnelian gems.
- Pliny the Elder (23 - 79 CE) in his famous work "Natural History" gives recipes for oiling and dyeing gems.
- An early "consumer advocate", Camillus Leonardus, an Italian physician and scholar, in a work called "Mirror of Stones" published in 1502 gives tips on spotting treated gems, like using a file to test for hardness, and hefting a gem to determine its specific gravity.
- By 1932 a gemological paper had been published listing fourteen known heating treatments.
Just as is true today, some of the motives of the gem treaters were honorable, and some were not.
The surveys that follow show a few examples (there are many, many, more) of some of the most common and economically important gem treatment processes. In some species the treatment is used occasionally, in others it is common, and in still others it is standard. The organization of the species presented, within each of these treatment type surveys, is simply alphabetical, not in order of dollar value, or frequency of use.
Heating: (AGTA Code = H) The most versatile and widely used treatment for gems is heating. Depending on the gem and the desired effect, temperatures used vary from those provided by placing the gems in direct intense sunlight, to near melting point temperatures of 2000 degrees C; periods of heating range from minutes to several days, and oxygen may be present or excluded from the heating atmosphere.
The atmosphere in which the gem is "baked" is important, as it will influence whether its ions gain or lose electrons. That is, it will determine if a chromophore ion will be changing from Fe3+ ---> Fe2+ or vice versa. A "reducing" atmosphere (one without oxygen) which can either be supplied via a high tech furnace, or simply by placing the gems to be treated in a closed container with charcoal), causes the number to go down (+3 to +2 for example). In an "oxidizing" atmosphere (oxygen present) the number goes up. (**You will recall from the discussion of the causes of gem colors in Lesson 4, the importance of the ionic state of the chromophore.)
Heating amber: Amber is heated for three main purposes: to darken it, to clarify it, and to deliberately add stress fracture inclusions.
When heated at low temperatures the surface of amber gradually darkens over time. Much of the clear amber found in nature is a light yellow to gold color, but shades from tan to gold to orange to dark brown can be obtained by heating. The color is usually confined to a surface layer and so is often done after the gems have been fashioned. If desired, the surface layer can then be partially polished or carved away to provide contrast or create a design. (Similar low temperature heating of ivory has been used, by unethical antique dealers, to darken its surface and create the illusion of great age). Low temperatures must be used on these gems as due to their organic nature, they will char, melt or burn!
In its natural state, much amber is cloudy or milky, an effect caused by suspended air bubbles. (If you have ever seen "whipped" honey, the appearance is very similar). Heating such pieces in oil can clarify them to a great extent (solid inclusions, if present, will remain however).
Finally, when amber is heated in oil and then "quenched"(plunged quickly into a cold liquid), characteristic stress fractures, that look like flat disks, form. These have been euphemistically called "sun spangles", and to some tastes, are an attractive enhancement.
The brooch photo below shows clear and cloudy amber of a variety of colors. The carving illustrates the surface nature of the heated color (where some of it has been removed by the carving process), and the close up shows the "sun spangles" in a piece of amber jewelry.
[Amber brooch with a variety of heated and unheated amber cabs, heat treated amber carving, "sun spangle"stress fractures created by quenching heated amber] Heating beryl: Two species of beryl gems are commonly heat treated. Aquamarine and Morganite occur naturally in shades of slightly greenish blue, and slightly yellowish pink, respectively, but the "market preferred" colors are pure shades of blues and pinks. Heat is used to obtain these preferred colors.
(The temperatures necessary to accomplish the removal of yellow tones by changing one iron ion to another using a reducing atmosphere, are low, and therefore, generally leave no obvious signs.) Therefore, aqua and Morganite of pure blue or pink color should be assumed to have been heated, unless otherwise stated.
The change is subtle and hard to capture with a camera, especially in Morganite as its color tone is so light, but the images below may give a general idea of the effect.
[Unheated and heated aquamarines] [Unheated and heated Morganites: Morganite images, copyrighted, used with permission of http://www.mineralminers.com] Heating chalcedony: Of the many forms of chalcedony, carnelian is the only one which is likely to be heated.
The orangey brown color of carnelian comes from its iron oxide content, which, when unheated, is hydrated (chemically, it has loosely attached water molecules bound to it). This form of iron oxide is known as limonite and is yellow to orange to brown in color. The amount of limonite which stains the chalecdony will differ, making carnelian naturally highly variable in tone and hue. Heat removes the bound water from the limonite and converts it to the unhydrated form, hematite, which, as its name suggests, is blood red in color.
Due to the low temperatures involved (the Ancients simply put it in the sun to bake) it is not possible to discriminate natural heating which might occur underground during, or after, gem formation, from that which is man-made. Therefore, if the color is significantly on the red side, assuming it to be heated, is erring on the side of caution.
[Carnelian briolette beads showing variation in natural color due to variable limonite content, unheated carnelian cabochon, a pair of very red carnelian cabs, which should be assumed to be heated] Heating corundum: Virtually all corundum gems (sapphires and rubies) have been heated. Many different outcomes from the heating processes are possible depending on the temperature, atmosphere, and the particular chemistry of the material being treated.
Interestingly, in corundum, heat: 1) can either increase or decrease color intensity, 2) it can dissolve rutile to clarify a piece, or 3) exsolve it to create or emphasize chatoyance or asterism. 4) It can be used to partially heal fractures improving clarity, and 5) it can diminish the tell-tale appearance of "curved striae" in synthetics by paritally melting these layers into each other.
We learned that heating can remove yellow tones in aqua, but by changing the conditions, it can emphasize them in sapphire. By using high heat and an oxidizing atmosphere, a pale yellow sapphire can acquire a deeper, richer color.
Blue tones in corundum can be increased or decreased: which way it goes is controlled by altering heating and atmospheric conditions. High temperature and rapid cooling under reducing conditions can change the ions of iron and titanium in pale blue sapphires to a form which results in a stronger blue color, for example.
On the other hand, some corundum suffers from too much blue color --> like certain quite purplish rubies and those "midnight" sapphires, so dark they virtually look black. Some of these gems are susceptible to conditions (oxidizing at high temperature) which removes some of the blue, making them much more attractive and saleable.
[Various color altering results of heat treating corundum gems: deepening yellow, deepening blue, lightening blue, removing blue, therefore correctlng purple to red] Corundum is also heated to change its clarity status. This is accomplished in two ways. Rutile is a mineral which, depending on conditions under which the gem was formed, may be dissolved within the corundum, and therefore not visible to the eye, or may have crystallized within the corundum as discrete needles affecting clarity and the chatoyance phenomenon. "Silky" corundum can be heated and cooled under precise conditions which will cause the rutile needles to dissolve into the corundum thereby greatly clarifying the gem; or conversely, gems with significant dissolved rutile can be subjected to heat and temperature regimes which encourage the dissolved rutile to "exsolve" into solid needles.
**Check the text: On pg. 94 of the Hall text there is a microphotograph of just the type of "silky" ruby that would benefit from dissolving its rutile needles -- in this case they are too few for good star potential, so dissolving them would make for a more beautiful and valuable transparent stone.
Due to the possibility of clarification, an increased percentage of potentially chatoyant corundum is now sold as transparent material, and the number of available star gems has decreased.
Gems with significant fracturing that causes loss of transparency can be subjected to very high heat which, by melting the thin edges of the fractures causes partial healing and, therefore, clarification to occur.

[A ruby whose originally modest star potential was enhanced by controlled heating, a pair of once silky blue sapphires, clarified by heating] ** Check the web: This company's website shows their high tech corundum treatment furnace and a couple of cool before and after pictures: http://www.swdgems.com/HeatTreatment.html
Evidence for heating in sapphires includes discoid stress fractures, singed (partially melted) surface facets, internal crystals with rounded, melted edges, and partially reabsorbed silk.
Evidence against heating is shown by intact silk, highly angular included crystals, and lack of discoid fractures.
[Heated for sure: discoid fracture in sapphire: Image courtesy of Martin Fuller, proof of no high temperature heating: intact silk in a sapphire] Most corundum gems cannot be called either way, so it is prudent to assume heating, as it is so prevalent in the marketplace.
**Check the web: Heating sapphires is SO common that even this company which calls itself "All Natural Sapphires" and boldy disclaims using gem enhancements can only go so far as to claim that "almost all of its sapphires are unheated". http://www.thenaturalsapphirecompany.com
Heating diamond: After diamonds have first been irradiated to green and blue green, they are often heated (a process termed "annealing") to further alter their color. Generally, such stones change to yellow or brown, but, rarely, some pieces with slightly different chemistry or crystallography, heat to highly desirable pink, purple or red colors.
The brown diamonds shown below are examples of annealed stones. The second photo shows an irradiated blue stone before and after annealing. The annealing takes place at relatively low temperatures (well within the range of that produced by a jeweler's torch).
Inadvertent cases of annealing have taken place when jewelers neglected to remove irradiated blue or green diamonds from their settings before repairs were made --> customers were not happy to find their diamonds had turned yellow or brown.
[Diamonds that have been irradiated then heated to change their color, the brown stones were deliberately changed, the yellow one was an accident] A New Process to be Aware of: A new high pressure/high temperature process for color enhancing diamonds is beginning to impact the market. It uses the same equipment and conditions that are have long been used to synthesize diamonds, but the treatment time is far shorter than that used for synthesis. AGTA (Code = HP).
Called HPHT for short, it can produce colorless, pink, and blue gems from some types of off-color rough or cut stones. Due to the very high temperatures used, only high clarity stones can be treated.
Not all diamonds react to this treatment, but when they do the results are stunning and the value of the stone jumps significantly. About 5% of diamonds can be made colorless, and a larger percentage can be made into "fancies". The fancy colors, are more subdued and natural-looking than those produced by irradiation, and unlike the case with irradiated stones, there are no obvious signs to help identify them as enhanced, so laboratory analysis is required.
[HPHT "Press" used by Sundance, Inc. to color treat diamonds, an example of a "before and after" showing a dramatic improvement in color: Images courtesy of Sundance, Inc.] Although Sundance, Inc. is doing business honestly, and attempting to prevent fraudulent representation of its goods, you can see, I think, how tempted some might be to try to pass off these relatively inexpensive enhanced gems as the higher priced "real thing". Heating quartz: As with corundum, heating quartz can have various effects. Gentle heating of dark or muddy amethyst lightens the purple, and can reduce unattractive grey and smokey tones. At higher temperatures amethyst converts to yellow or orange citrine or, rarely, to yellow-green prasiolite. (Chemical or physical factors present in amethyst mined in only a few sites are of the sort that create prasiolite when heated, so it is much rarer than citrine. Citrine does occur naturally, in which case Nature has already supplied the heat, but in general, natural color stones are notably lighter in tone than those produced with human help.
Smokey quartz when heated can turn yellow, also making citrine although this is less commonly done than heating amethyst. Tiger'seye which is usually a golden yellow color will become red upon heating.
[Various outcomes of heating amethyst: lightened amethyst, citrine, prasiolite] [Unenhanced citrine] [Heated quartzes: citrine from heated smokey quartz, enhanced red tiger'seye] Heating topaz: Two types of topaz are routinely heated. 1) White topaz, as a first step in its color enhancement, is irradiated to brown, and it must then be heated to create a stable blue color. 2) Much natural ("precious") topaz of a yellow or orange color, some with subtle pink overtones, is unattractively muddied with brown, which can usually be removed or reduced by heating, a process traditionally referred to as "pinking". As can be seen in the photo of the four stones on the right below, the results are variable.
Both processes use relatively low temperatures, so there is little evidence left behind. Once again, it is prudent to assume that any blue or precious topaz has been heated unless it can be proven otherwise. Natural blue topaz is very rare, and when found it is generally quite pale, and pinking of precious topaz rough is standard practice virtually everywhere that it is mined.
[Heated (sky, Swiss and London blue topazes): blues are heated post-irradiation, "pinked" precious topaz] Heating tourmaline: Heating can be useful in lightening the color of some dark blue and green tourmalines that without such treatment, look almost black. Unfortunately, not all dark stones respond to the heating. In some cases, as with amethyst, muddy tones also can be lightened or removed. For these reasons, the majority of blue and green tourmaline rough is heated, so it is prudent to assume it. Some red tourmalines (rubellites) can be improved in color by heating, and though not common practice, it is occasionally done.
[Heated blue and green tourmalines, lightened enough to be attractive, a red tourmaline that might possibly have been heated] As with topaz, the temperature used for tourmaline is fairly low, so there are few telltale heat-altered inclusions to leave evidence of it. Such stones, however, can be more brittle than unheated ones which can sometimes be deduced by noticing facet junctions that are abraded more easily than the norm.
Heating zircon: Zircon which occurs naturally in orangey brown shades, has both a long history of use as a gem, and a long and creative history of enhancement by heating.
** Check the text: Page 73 of the Hall text has a very nice picture of a step cut rectangular unheated stone that shows the natural color very well.
The same stones are sometimes put through a series of heat/atmospheric enhancement regimens in an attempt to induce a change to a more desirable color such as blue, blue-green, red, yellow, orange, or red. Individual stones (based on their own unique chemistry) react in various ways. The description that follows applies to the majority of zircon gems:
In the first step of treatment, rough zircon is exposed to temperatures of around 1000 degrees C, in a reducing atmosphere where many brown stones turn blue, some turn white and others don't change.

[1st Round: before, brown, and after: blue or white] Those which do not respond may then be re-treated at slightly lower temperatures of about 900 degrees C, in an oxidizing environment. Results can vary from yellow to white to red.

[2nd Round: before: brown stones which didn't respond to initial heating, after: yellow, white, or rarely, red] Stones may sometimes go through several cycles of heating, and can get rather brittle, which may make facet edges susceptible to abrasion.
Although shades of orange, red and yellow are occasionally found in Nature, white and blue occur so rarely that heating must be assumed for these colors.
Heating zoisite: When a transparent variety of zoisite was first found in Tanzania, Africa, there was little excitement due to its dull, brownish yellow color. Experiments with heating, though, soon yielded gems of a beautiful blue-violet color. As it turns out, heating was turning one of the color axes of this naturally trichroic gem from yellow-green to colorless, and allowing the less dominant blue and violet colors to be clearly seen. "Tanzanite" was born. As is so commonly true of gem rough, some individual pieces have atypical chemistry or crystallography, and react differently to treatment than most. In the case of this type of zoisite a very small percentage of the gems heat to an attractive green to blue green color. Dubbed "Green Tanzanite" (a misnomer, but one that stuck), such specimens have high value as collector stones, and are quite beautiful in their own right.
[Typical unheated color of Tanzanian zoisite, the usual blue-violet result of heating, the rare green result]
Help! I want my gems unheated!There are two major groups of gems that aren't heated: 1) heat sensitive gems like opal, apatite, pearls, and turquoise, and 2) those for which heating makes no improvement, or isn't economical such as garnet, spinel, chrysoberyl, iolite, peridot, sunstone, moonstone, jade, and most collector stones. If unheated is what you want, that is fine, but outside of the groups listed, you can expect to pay a premium for unheated goods, and some types of gems like blue zircon and Tanzanite will be off limits entirely.
Another point to keep in mind is that "unheated" doesn't mean the same thing as "unenhanced", although there are those who can profit from implying it does!
I have seen several dealers on independent websites, online auctions, and at gem shows that proudly advertise their wares as "unheated" when their goods have been, dyed, coated, irradiated, oiled or in some other way enhanced. Naive buyers can be deluded into thinking they've purchased an unenhanced stone.
Irradiation: ( AGTA Code = R) After heating, the most commonly used treatment for gem enhancement is irradiation. With some important exceptions (like diamonds), treated stones are usually not distinguishable from untreated ones, as gems are often subject to similar, but natural, irradiaton effects during, and after, their formation.
A variety of sources of, and processes for, irradiation have been tried, some, proving unsatisfactory, have been abandoned, others are still in use. The earliest experiments with irradiating gems used alpha particles (helium nucleii) which worked as desired, but left the gems with strong and highly persistent residual radiation. Today, beta particles (electrons) generated in linear accelerators, neutrons from nuclear reactors, and gamma rays usually from radioactive cobalt sources are used.
Although the bombardment with neutrons and, to a greater extent, with electrons, can leave some residual radioactivity, its duration is relatively short. Government agencies in the USA, and other gem irradiating nations, have strict regulations for the holding and testing of irradiated gems to assure that they are not released to the public until they are safe to handle and wear.
As we saw to be the case with heating, different types and durations of irradiation produce different results. Combining this fact with the idea of individual variation in gem chemistry, and that irradiation may or may not be followed by some sort of heating process, and we can begin to appreciate the tremendous diversity of possible results.
Irradiating beryl: Colorless beryl (variety = Goshenite) can be irradiated to stable shades of yellow to gold (variety = golden beryl or heliodor). Unenhanced golden beryl is also common, and it is essentially impossible, outside of a large gemological laboratory, to tell whether it was man or Nature that supplied the color producing irradiation.
[Goshenite, golden beryl] A boondoggle perpetrated on the gem buying public several decades ago, is still remembered by some wary dealers and buyers. Some beryls, with an unusual chemistry, turned a vivid and attractive blue when irradiated. They were rushed into the market with much fanfare under the tradename "Maxixe" beryls. The fly in the ointment was that these stones were unstable in light, and reverted to their intially colorless state relatively quickly under normal use conditions.
There would be little point in bringing up this unsavory memory, except that in 2003 explorers in Canada unearthed a bright blue deposit of beryl reminiscent of the Maxixe color. This material is colored by iron (not irradiation), and the color is stable. As of yet, the mining efforts have not yielded any facet quality rough, but exploration is proceeding. The only known deposit is presently owned by "True North Gems" and their material, already popular with collectors, has been christened "True-Blue Beryl".
[Unstable irradiated "Maxixe" beryl, natural color, stable, "True-Blue Beryl": Image courtesy of True North Gems] Irradiating diamonds: As presented in the section on heating gems, diamonds irradiate to green or blue green. Another color which is commonly produced via irradiation is "black". Well, actually, it is not black but a very, very, dark green, and the visual impression is definitely black.
Diamonds do occur naturally in black (if they are highly included), but irradiated "black" diamonds, when subjected to very concentrated light source, like a fiber optic light, show green color at the extreme edges where the girdle or facets are thinnest, whereas this is not true of unenhanced black diamonds. Virtually all the black diamonds used in jewerly today are the irradiated type.
[Typical blue-green color of many irradiated diamonds, very dark green "black" irradiated rose cut diamond] You will recall that these irradiated stones can then be annealed (heated) to brown, yellow or, less often, some of the rarer fancy colors like red. These heated and/or irradiated colored diamonds tend to have more vivid (some might even say "garish") colors than naturally colored fancies.
Unlike what is seen with the majority of species which are irradiated, the process does tend to leave tell-tale signs in most diamonds. Patterns of color zoning that show up microscopically, with certain lighting conditions, can usually give the trained observer evidence of the treatment.
With enhanced blue colored diamonds, though, there is a definitive test--> electroconductivity. Diamonds that come by their blue color naturally contain boron impurities which allow them to conduct electricity, irradiated blues get their color by the effect of irradiation on their crystal structure, and like all other diamonds, don't conduct.
Irradiating pearls: One of the many possible enhancement processes that are used to change color in cultured pearls, the use of gamma irradiation has different effects on fresh and saltwater cultured pearls.
When saltwater pearls, like Akoyas, are irradiated, it turns the bead nucleus (made of shell) dark, which then shows through the unaffected translucent nacre layer making the pearl look grey or perhaps grey-blue. In the case of cultured freshwater pearls, the irradiation actually affects the nacre layers creating silver, gold, black and even multi-hued, often intensely metallic looking colors. (This difference is due to the slightly different trace element chemistry of nacre produced in fresh vs saltwater.)
The treatment in both cases is stable. With saltwater pearls it is usually possible to get a magnified view down a drill hole which will reveal the darkened bead nucleus. Identifying irradiated freshwater cultured pearls (which usually have no bead nucleus) can be as simple as becoming familiar with the natural color range of these gems. Once that is accomplished, the completely unnatural looking irradiated stones jump out at you and identify themselves!
[Irradiated freshwater cultured pearls: Image courtesy of www.earthstone.com] **Check the web: This GIA report was done shortly after the 9-11 incident. At the time, some letters and parcels were being irradiated by the US Postal Service as a counter bio-terrorism measure. The before and after pictures show how sensitive pearls are, even to low levels of radiation: http://www.gia.edu/newsroom/608/384/news_release_details.cfm Irradiating quartz: Colorless quartz, rock crystal, is irradiated to produce smokey quartz in shades from light to very dark brown or grey-brown. The smokey quartz found before modern irradiation techniques were developed, and a proportion of that mined and sold today, comes pre-irradiated by Mother Nature.
A relatively recent discovery of a unique type of rock crystal at a particular series of mines in Brazil gives quite different results when irradiated. The resulting material known variously as "neon" "lemon" and "oro verde" quartz has a highly saturated, slightly greenish yellow color. Due to its striking color and its availability in inexpensive, large, clean pieces, it has become the recent darling of carvers, concave facetors and fantasy cutters, as well as a staple of the home shopping channels, and internet gem auctions.
Once again, with smokey quartz, we have a case where it is difficult to impossible to determine the origin of its color, and it is best to assume it has been irradiated by man, in the absence of proof to the contrary. Not so, with the yellow material, as its only known source is from the irradiator's factory.
[Rock crystal quartz, smokey quartz (could be natural color or irradiated), "oro verde" quartz (always irradiated)] Irradiating scapolite and spodumene: Although these two gem species are likely to be unfamiliar outside of gem circles, they provide some interesting examples of the effects of irradiation. Scapolite is found naturally in light to medium shades of yellow, and also in pale lavender. The yellow material can be irradiated to a much deeper, and more brownish, shade of lavender than that which occurs naturally.
Spodumene occurs naturally in colorless, light to medium pink/lavender (Kunzite), and very rarely in a chromium colored, grass to emerald green stable variety known as Hiddenite. Kunzite can be irradiated to a light to medium green color which is often given the misnomer of Hiddenite, and sold for inflated prices to naive collectors. Not only is the irradiated material not Hiddenite, as its color does not come from chromium, but it fades quite noticeably in the light, a fact too rarely included in the sales pitch.
**Check the web: This web page from the North Carolina Museum of Sciences, shows and discusses the very rare gem Hiddenite with a photo of the original specimen collected by William Hidden in 1905: http://www.naturalsciences.org/funstuff/notebook/geology/hiddenite.html [Yellow scapolite, irradiated purple scapolite, Kunzite (pink spodumene), irradiated green spodumene: image courtesy of www.faceters.com] Irradiating tourmaline: The world's supply of attractively colored pink to red tourmaline has recently been greatly increased by new discoveries. Some Brazilian tourmalines, formerly rejected due to poor color, and the majority of the large new deposits being found in Africa, are irradiated to diminish brownish tones. The permanent improvement in appearance (alas, not all pieces are susceptible) is similar to that displayed in the two stones below:
[Typical "before and after" colors of some low grade tourmaline that is susceptible to drastic improvement in color after irradiation ] Irradiating topaz: In terms of sheer carat weight, and probably also in terms of economic value, blue topaz is the most important irradiated gem. Colorless topaz is plentiful and inexpensive, but not all of it will irradiate successfully. Treaters generally screen the rough with an inexpensive gamma ray treatment which identifies the rough which will benefit from further irradiation, before proceeding with more costly and time consuming treatments.
These "good candidates" then go to either a linear accelerator to be bombarded with electrons, or to a nuclear reactor to be exposed to neutrons. Depending on the duration and type of irradiation, and the sort of heating process used afterward, the results vary from sky, to Swiss to London blue. Other slight color variations have been produced and given their own tradenames like "electric blue" and "neon blue".
London blue is the scarcest and most expensive type because it requires neutron exposure (most expensive process), and the longest holding times. Now that so many other types of gem materials are being irradiated, topaz treaters are having to pay more to "book" accelerator or reactor time, and prices for these once quiet inexpensive gems have correspondingly risen.
With most gem materials, much, if not most, of the cost of the finished gem, is associated with the gem rough itself. Blue topaz is an interesting exception to this, as the rough is the least expensive part, with fashioning, and even more so, treatment, responsible for the bulk of the costs.
The irradiated colors are stable, and the gems are perfectly safe to wear, but the dual heat/irradiation processing does leave them somewhat more brittle than unirradiated stones. Added to the natural tendency for cleavage, this makes blue topaz one of those gems which, despite its hardness of 8, is not a good choice for daily wear rings or bracelets.
[Unenhanced white topaz, sky blue, Swiss blue, and London blue irradiated/heated topazes] Unintended Consequences! The introduction of huge amounts of blue topaz into the market place in the last several decades has had some interesting side effects on other gemstones.
1) For a time, it depressed the prices of aquamarine, as sky blue topaz made a pretty good aqua simulant at about 1/10th the price. With time, though, most aquamarine lovers went back to their original gem choice, with the ironic twist of a noticeable decrease in the traditional preference for heated "pure blue" stones. More and more aqua fanciers are now seeking out the greenish blue unheated stones--> possibly because these are less likely to be mistaken for the ubiquitous and inexpensive blue topaz!
2) The other effect has been to literally wipe out the identification of the word topaz with the color yellow. Yellow, or precious topaz, was, until the advent of irradiated blue, the most common and familiar type, and is still the traditional birthstone for the month of November.
[For centuries, these were the colors that the word "topaz"evoked!] Food for thought: (Answers to the questions are found at the end of the lesson)
Question 1: Your friend wants an untreated gemstone and has found a dealer with some beautiful golden beryls. The dealer assures him that they have not been heated. He asks for your advice. What do you tell him?
Waxing: (AGTA Code = W) When the surface of a gem is coated with colorless wax, (or oil) the process is termed waxing. Generally, this treatment is used with stones with a vulnerable, porous surface, or those with microscopic surface imperfections whose polish luster can be boosted with it. Porous materials like turquoise that have been waxed, are thereby, at least partially, protected from absorbing skin oils and other environmental contaminants.
Although, not permanent, the gem community tends to adopt an extremely forgiving attitude toward this treatment, as it is both a very long standing tradition, and a simple matter to re-wax the gem. (Paraffin and beeswax are the traditional materials used, and re-doing a gem can be as simple as painting on melted wax and buffing off the excess).
Most of the world's highest grades of turquoise and jadeite can be assumed to have been given this treatment. It is also occasionally used with lapis lazuli, rhodocrosite, serpentine, variscite and Amazonite.
[Persian grade, waxed turquoise earrings, waxed "A" jadeite, moss-in-snow cabochon, waxed lavender "A" jadeite ring: Image courtesy of Mason-Kay, Company] Dyeing: (AGTA Code = D) Dyeing is relatively easily accomplished with porous gems and those crystalline gems which are aggregates. The pores and the spaces between the microcrystals allow the dye to be taken up. Single crystal gems, however, are not good candidates for dyeing as they will only take up dye where they have surface reaching fractures.
There is really only one case in which dyeing is an "accepted industry standard", and has no effect on the value of the gem: black onyx. All other instances of dyeing (when disclosed) negatively affect the value of the gem, in some cases, dramatically.
Examples of porous and aggregate gems which are frequently dyed are chalcedony, jade, coral, pearls, and howlite.
There are some cases where the absorption spectrum of a gem can identify specimens which are dyed, but they are rare. The usual ways of detecting dyed gems are:
1) By microscopic examination: looking at pores, bead drill holes, and surface fractures for dye accumulations.
2) Testing with a solvent, a destructive test, yes, but one which can usually be done in an inconspicuous area. Useful solvents are acetone and denatured alcohol, but not all dyes are soluble in them, so a negative test in not conclusive.
3) Comparison to the normal range of gem colors and evaluation of the avialability and cost --> naturally colored chalcedony in unknown in hot pink for example, and Nature doesn't yield neon green pearls. Saturated, medium dark, lavender natural color jade is worth a King's ransom, so pieces of that color seen on ebay for $10 are very likely to be dyed. (Compare the color of the inexpensive jade beads below with the picture of the very expensive natural color lavender jade ring above).
[Dyed "C" jadeite green earrings, and lavender beads: image courtesy of www.mbbeads.com, a microscopic view of dye accumulations in jadeite: image courtesy of Martin Fuller] [Dyed coral beads, dyed freshwater cultured pearls: image courtesy of www.mbbeads.com] [Typical natural color of chalcedony, typical non-natural color achieved by dyeing] One of the most commonly dyed materials in today's marketplace is howlite, an inexpensive, porous, white mineral that generally has grey to black veining. It has been used to simulate turquoise, lapis, rhodonite, and other opaque gems. The dye penetrates only a short distance into the gem, so scratches and chips are revealing, but when undisturbed, a piece can be quite convincing.
[Howlite in its natural color: image courtesy of Bill Wise, dyed to imitate lapis lazuli: image courtesy of www.earthstones.com, dyed to imitate a turquoise nugget, the nugget sawn apart to reveal the undyed interior] When single crystal gems are dyed, they must be fractured first. In order to achieve this, the age old method is "quench crackling". Generally this is done by heating the gem, and plunging it in cold water, but strong ultrasonic vibrations have been used to accomplish the same thing. Dye can then be absorbed into the fractures which, when numerous enough, give the piece an overall color.
[Two pieces of quench crackled, dyed rock crystal quartz, the pink one has been magnified and shows clearly that the dye is only in the fractures] In the great majority of cases, the dye is a chemical or pigment of either natural or synthetic origin. An example is the use of silver nitrate, a chemical that darkens on exposure to light, which has been used on pearls for many years.
There are a couple interesting cases, however, which involve "carbonization". Chief among these is the production of "black onyx". Here's one of those cases where a misnomer continues in use, just because of familiarity and convenience. (Onyx by definition is has color bands, so a solid black material just doesn't qualify). There are very small amounts of black chalcedony found in Nature, but the virtually all of that in commerce is carbonized chalcedony.
Colorless to light grey chalcedony is soaked in a sugar solution until its internal pores are filled with it, then it is boiled in sulfuric acid which "carbonizes" the sugar turning it black. There are now microscopic sized black specks throughout the piece, giving it a uniform and stable black color. When I was first learning about gems, I just couldn't believe that this primitive method, developed hundreds of years ago, was still the major mode of production...but it is!
["Black onyx" ring, technically "carbonized chalcedony"] **Check the web: Here's a web page with a quite detailed "recipe" for making black onyx http://www.ganoksin.com/borisat/nenam/black-dying-agate.htm A similar process is used to color certain matrix opals (particularly those from Australia's Andamooka region), whose matrix is a light color, giving little contrast to the patches of color of the opal within the matrix. The result of the darkening of the matrix is an improvement in contrast and therefore in the appearance of the color play.
Bleaching: (AGTA Code = B) Probably the most routinely bleached gem, is pearl. Historically, long before cultured pearls were invented in the early 20th century, pearl fisherman would spread their treasures out in the bright sunshine, carefully rotating them over a period of time, which tended to lighten and even the color, and diminish some unsightly dark spots. Light is still used in some pearl processing facilities. (Anyone whose hair gets lighter in with long exposure to sunshine, or whose window drapes have faded over time, will realize how effective a bleach light can be.
Besides its use in removing blemishes and evening out color, bleaching is a tremendous aid to manufacturers in matching pearl colors. Today's pearl consumer demands that each and every pearl in a strand, is exactly the same shade---> Mother Nature prefers to make a wide variety of shades, even within the same species of oyster or mussel, living in the same body of water.
In addition to light, chemicals such as hydrogen peroxide (Lady Clairol, anyone?) and chlorine (as in Clorox bleach), speed up the process, but on delicate organic gems like pearl and coral, must be used at low strength and with care.
[Large scale bleaching of pearls with chemicals and/or under lights: Images courtesy of www.man-sang.com] Golden coral is rare and valuable, so is black coral. Depending on the ups and downs of the market, the vagaries of supply, and prevailing public tastes, there are times when black coral is bleached to gold (peroxide is used).
[Bleached black coral beads: Image courtesy of www.stonesnsilver.com] Jade is another frequently "bleached" gem, but in this case strong acids are used which really aren't bleaching the color to a lighter shade, they are literally dissolving away discoloring inclusions. Such bleached jade requires further treatment to seal the cavities formed by bleaching. (See below.)
Acid bleaching is also used in conjunction with laser drilling in diamonds to remove "carbon" spots and other discolorations. The laser creates a narrow channel by which the acid can penetrate the interior of the diamond and do its work. As with jade, this process is generally followed by one which "fills" the cavity. (Again, see below)
Impregnation (aka "stabilization"): (AGTA Code = I)
When a colorless, hardened, resin is suffused throughout a porous stone to make it more durable or improve its appearance, it has been impregnated. A common market term for such gems is "stabilized". There is only one imporant type of gem for which this treatment is essential--> without impregnation, ammolite is too fragile to withstand fashioning or wear. Unenhanced specimens, exist, but are suitable only for display.
Other gems like jade or turquoise are commonly treated in this manner. Low grades of highly porous turquoise that may have nice color, but are excessively fragile, or near impossible to polish, can be greatly improved by resin impregnation. "B" and "C" jades, after being acid bleached have resin infused into the resulting cavities (if the resin is colored, then the piece is considered dyed, "C" jade).
[Impregnated gems: ammolite pair, turquoise cabochon, "B" jade ring] Oiling: (AGTA Code = O) and Filling: (AGTA Code = F) Both of these types of treatments involve the filling of surface reaching fractures or cavities with colorless oils, resins, or glass. They are done for the same reason: to clarify a gem, by decreasing the relief of the fracture or cavity. The difference between them hinges on whether the filling material is essentially a liquid (oil or unhardened resin) or solid (hardened resin or glass).
Of the two, oiled gems are more accepted in the gem marketplace and do not depress value greatly. Even though the oil treatment is temporary, the favorable viewpoint comes both from the long standing and widespread use of gem oils, and the fact that it can be successfully be re-done if necessary. Virtually all emeralds are oiled, some as rough at the mine site, others only after they are cut. Certified unoiled emeralds bring a 10% - 20% price premium. If the oil is colored, then the emerald is considered to be dyed, and its price is much more severely affected.
[Emeralds: routinely oiled to improve clarity] Filled gems are another matter. Although it can be argued that the filling, being hardened, is less likely to evaporate or be dislodged by cleaning and wear, several factors create a generally negative impression that translates to a drastic effect on gem prices.
The solid resins can discolor and become more opaque with age, and since they cannot be removed, will then permanently degrade the gem's appearance. Fairly large areas can be filled with solids, which are less durable than the host gem and can become scratched, chipped or dulled with wear. This process is primarily used with rubies and diamonds, both very valuable gems, so it must not be forgotten that these chunks of glass or plastic resin are adding weight to the gem. That is, adding weight of a material which is not valuable, but which the customer is paying for.
Until just recently, diamonds were the only major gem for which glass filling was a concern. Now, however, the number of glass filled rubies in the marketplace has risen to the point where gem professionals and alert buyers need be wary. Glass or plastic filled gems are worth only a small fraction of the value of their unenhanced equivalents.
Fortunately, there are several ways to detect glass or plastic areas in gems. Under reflected light, the luster difference between a ruby or diamond, and its glass filler are easy for the trained observer to spot. Some fillers fluoresce and give themselves away. For those which are confined to thin fractures, the "flash effect" is a tell-tale sign.
The Flash Effect: As a gem is rocked and tilted under appropriate lighting and magnification, these thin filled areas will first flash one solid color like orange and then, at a different angle, a different color like purple. Novices may confuse this with the cleavage rainbow seen in unfilled fractures, but in the flash effect, only one color is seen at a time.
[Fracture filled diamonds showing the "flash effect": Images courtesy of Martin Fuller] Gems which are properly disclosed as oiled or filled, should include appropriate care instructions. For example, oiled emeralds should not be steam or ultrasonically cleaned. Jewelry containing filled rubies and diamonds should not be repaired or resized without removing the gems from the settings, as heat from a torch or immersion in metal cleaning solutions can cause melting or etching of the filler.
Food for thought:
Question 2: You walk into a jewelry store with your brand new gift ruby ring, and the emerald brooch you inherited from Aunt Minnie. You want the ring sized, and a new closure soldered onto the brooch. You ask the jeweler for the estimated charges including dismounting and remounting the gems. She says dismounting will not be necessary. What should you do?
Laser drilling: (AGTA Code = L) Thus far, this treatment, a type of clarity enhancement, has been seen only in diamonds, and is virtually always combined with acid "bleaching" and fracture filling. The purpose of the tunnel created by the laser is to provide a channel for the acid and glass, or resin, to enter. The entry points are tiny, as seen in first photo (red arrows) and can be easily overlooked, but microscopic examination (usually 10x is sufficient) makes this treatment one that can be positively identified.
[Laser drilled diamond, surface view and magnified view: Images courtesy of Joe Mirsky, laser drilled diamond, note flash effect being shown by filled fracture at the base of the laser tunnel: Image courtesy of Martin Fuller] Diffusion: (AGTA Code = U) When gems are diffused, they are heated to very high temperatures, just to the verge of their melting points. This heating is done in the presence of a material which contains chromophores such as titanium, chromium or other atoms, which are then able to diffuse into the stone's surface or interior to change color or create phenomena. Two such processes are currently in use: 1) surface diffusion, and 2) bulk or "lattice" diffusion.
Surface diffusion has been around for decades and, until recently, was pretty much confined to use on blue sapphires and the occasional ruby. By packing already faceted, light colored stones into a container with powdered titanium and iron, and heating to very high temperatures, a thin surface layer rich in these chromophore elements is formed, which through selective absorption, greatly darkened the apparent blue color.
Such stones must always be repolished afterward as the high heat tends to mar the surface. In the repolishing process it is inevitable that some of the thin layer is unevenly removed, so that when viewed under immersion and/or in diffused light, an uneven pattern of color--> paler on some facets than others and darkest at the edges of the facets, can be seen.
If the diffused stone has inclusions at all, these will also show the typical signs of high heat, such as partially resorption of silk, partial melting of crystals, or stress fractures. With such obvious signs of treatment, only the unschooled or unwary buyer is likely to be duped.
Below is a picture of a 1.53 ct. sapphire. A beautiful stone: top color, eyeclean, and reasonably well cut. In today's market one might expect to pay between $1000 and $1500 per carat, retail, or even more in an upscale jewelry store for a sapphire with this kind of appearance. This stone's actual retail price was $150 for the piece, or less than $100 per carat. What a bargain, you say --> and why so cheap? (No, it is not a synthetic!)
[An attractive, color enhanced, natural sapphire] The price paid, was, however, appropriate to the stone. It is a natural origin sapphire, but it is has been color enhanced by surface diffusion. The lovely color layer has a thickness of much less than one millimeter, the rest of the stone is either colorless or an unappealing pale blue or grey.
Such stones represent a bargain, as long as the customer understands the limitations inherent to them. Any scratch, chip or nick will remove the color layer revealing a light spot, and the stone cannot be recut or it would lose its color entirely. As a stone to be used in a pendant or earrings or even a ring worn once in a while, it will look beautiful for many years, but it is not an appropriate choice for a frequently worn ring or bracelet.
You can see from the photo, that looking at the stone in ordinary light doesn't tell you the source of its color. Below are two 10x magnified photos of this same gem, (you can do this kind of observation with your loupe, no fancy microscope is necessary).
In the first, the gem is seen in diffused light, in the second, it is immersed in water and viewed with diffused light. Both photos give unequivocal evidence of surface diffusion. Look closely and note the color differences from facet to facet (natural color zoning does not follow facet shapes), especially telling is the way the color is darker along the keel and at many of the facet boundaries.
[Magnified views of a surface diffused blue sapphire: under diffused light, under immersion in water with diffused light] Making Stars When titanium dioxide (rutile) is surface diffused into a sapphire, and the heating and cooling is controlled so that it exsolves into needles, asterism is created in the stone. Again, you are looking, in the photo below, at a natural sapphire, but one that has been surface diffused with rutile. Such gems are very inexpensive compared to unenhanced, natural star sapphires. In comparison to an unenhanced star stone, the star figure is stronger, more even, and seems less mobile as the light source shifts direction.
[A surface diffused star sapphire] A recent entry into the world of surface diffused gems is topaz, now available in bright colors such as green and red as well as bi-colors. The advertisement below is from an ethical company (RioGrande), which is one of the major suppliers of gems to jewelers: notice the enhancement code (U) and the appropriate warning about recutting.
[Graphic courtesy of RioGrande Corp.] In 2004 Leslie and Company introduced the first surface diffused bi-colored topazes to complement their existing line of tradenamed diffused topaz gems.
[Surface diffused topazes: Images courtesy of Leslie and Company] Bulk or lattice diffusion was discovered to be occuring in 2003 when stones that began appearing the market in 2002 were critically examined. The gems were sapphires of an extremely rare and valuable color called "padparashah" (orangey pink). The inexplicably large numbers of fine colored stones suddenly available, raised questions that led to a fervor of activity among the staff gemologists in organizations such as AGTA and GIA.
Athough the treaters were freely acknowledging that the gems had been heated, they insisted that that was the end of the story. Adding to the confusion, the stones did not fit the profile of diffused gems as the color penetrated well into the interior--> in many causes completely throughout the stone.
Suffice it to say that they were finally demonstrated to be the result of a new diffusion process using the light element beryllium (Atomic number = 4) as colorant. With its small atoms, the beryllium chromophore was able to diffuse much further into the heated stones than titanium or chromium with their much larger atoms. The source stones were primarily light pink African sapphires which were then being were treated in Thailand.
[Bulk diffused "padparashah" sapphires] The "pre-discovery" prices were extremely reasonable for naturally colored, or simple heat treated paparashahs, but outrageously inflated for diffused goods. In the interim between the time the stones were stealthily introduced into the market, and the time their true nature was understood, quite a few greedy collectors and dealers re-learned the old lesson "If it seems too good to be true, it probably is".
Viewed under diffused light and /or immersion, most of these gems show normal color patterns. Although they do have inclusions typical of high heat, positive identification of a particular gem as being beryllium diffused, requires the services of a large gemological laboratory.
Although they are considerably more durable than surface diffused stones, recutting could still be a risky proposition, especially on larger stones.
Coating: (AGTA Code = C) Coated gems are those that have been treated with surface enhancements such as laquering, inking, painting, foiling, or sputtering of a film to enhance color, improve appearance or add phenomena.
Coating has a long history: from use of gold foils in antiquity, to the painted back Rhinestones of the 19th century, to today's iridescent metallic coatings. Coatings are usually fairly easy to detect, but can escape notice if they are applied only to back of the gem (as in a "foilback") and the gem's setting is fully closed. The coatings of foilbacks range from crude and obvious, to sophisticated and well hidden, as seen below.


[Contemporary "gumball machine" quality foilback ring (glass with metallic paint), circa 1910 high quality Rhinestone brooch (glass with metallic paint)] Perhaps the most nefarious of the fraudulent uses of coating is an old (but still in use) trick of putting a tiny drop of indelible blue ink or paint underneath the prongs holding an off-color diamond in its setting. The prong hides this dot of color from all but the most experienced eyes. The effect of the light reflecting off those blue areas and mixing with the generally yellowish light emerging from the gem, makes the yellowish stone appear shades whiter. (As an example, this technique might raise a stone's apparent color grade from M to F allowing the seller to make an undeserved profit.)
The most common coatings in today's gem market are the metallic vapor films that create iridescent gems.
[Metallic vapor coated iridescent quartz ("aqua aura"), magnified view of metallic vapor coated "mystic topaz"] Care of Enhanced Gems There are no overall rules, as some enhancements increase durability, while others decrease it. But these general precautions will protect almost any enhanced gem: avoidance of solvents, ultrasonic and steam cleaning, gentle wear, protective settings, avoidance of recutting, and removal of gems from their settings prior to jewelry repair.
Answers to the thought exercises for this lesson. (If you don't understand something in these answers it's time to email me and ask!)
1): You might make sure that your friend understands the difference between "unheated" and "unenhanced." Most likely he is under the impression that they mean the same thing. Few people who have not studied gems realize the vast number of treatment possibilities there are. You could also mention that a lot of the golden beryl in the market has been irradiated. A possible suggestion would be that he ask the dealer, point blank, whether the gem has had any enhancement at all. (On the other hand you might like to just keep your mouth shut, and smile, and agree that they are lovely).
2): The safest thing to do would be to walk right out and find another jeweler. The new ruby ring could be glass filled, the emeralds are almost certainly oiled--> in either case the heat from the jeweler's torch (even when methods are used to keep the gems isolated from the heat) can do major damage. Even if the gems were completely unenhanced, there is still the possibility of damage from inclusions due to thermal expansion.
You have now completed the web lecture for the eighth lesson!
Go back the the course website to: 1) complete and submit the homework assignment on the text readings and assigned web essays 2) take the non-graded practice quiz on this web lecture 3) post a comment to the discussion board for this lesson, and 4) when it is available, complete the graded quiz based on this web lecture.
When you're ready, proceed on to Lesson Nine: Synthetics and Simulants