Synthetics are man-made gem products. The Federal Trade Commission is quite specific in forbidding the use of the term "gem" or "gemstone" (or any recognized species or variety thereof), unless that product is solely and exclusively the work of Nature. All other products offered for sale must be clearly identified with a readily understood adjective that indicates their synthetic status. Acceptable terminology for synthetics is variable, but would include product labels similar to the following: "synthetic gemstone", "laboratory-grown ruby", "cultured pearl", "created emerald", "man-made sapphire", "reconstituted turquoise".
Synthetics can be exact copies of natural gems, or they can be unique materials which are not found in Nature.
1) Examples of the former include the synthetic rubies and emeralds. Such creations have virtually the same optical, chemical and physical properties as their natural counterparts.
[Synthetic, Chatham brand, emerald crystal cluster set as a pendant] (Also placed in this category are those created materials which are accurate copies of natural gems, except that they are produced in colors not found in Nature. Examples would be blue quartz, and white spinel-->except for color, these give all the same test results as the natural versions of that gem.)
[Synthetic blue quartz: quartz, yes, but in a color not found in Nature] 2) Man-made wholly "artificial" gemstones with no natural counterparts at all, include cubic zirconia (CZ) and YAG (yttrium aluminum garnet).
[YAG, a popular synthetic widely used in industry and as a gem, can be made in a variety of colors from white to pink to green, and has no analog in Nature] Check the text: See page 354 in the Lyman book for a picture and description of another artificial garnet GGG (Gadolinium Gallium Garnet) History
Unlike enhancements and fakes, synthetic gems are a relatively modern development. Although there were some successful laboratory experiments earlier, the economically viable commercial production of a synthetic gemstone began with synthetic ruby produced in France in 1902 by Auguste Verneuil. By 1907 production was 5 million carats per year.
The fact that synthetic gems were produced so early often comes as an unpleasant surprise to those who inherit or purchase a vintage jewelry item. The heir, or buyer, is expecting that the age of the piece, alone, guarantees natural origin. Although in more recent years synthetics are, at best, found in "entry level" jewelry, in the first two or three decades of the 20th century, synthetics were sometimes used by noted designers, and high end jewelers, who appreciated them as signs of "scientific progress" or "modernity".

Outside their use as synthetic or simulant gems, physicists and chemists make large quantities of both copies of natural gems, and totally artificial ones, for industrial and research purposes.
At present over 90% of the diamond abrasives ("bort") for industry, used in everything from the saws that cut through pavement, to dentists' drills, are synthetics produced in a laboratory. Laser and electronic technologies depend strongly on the properties of laboratory created crystals. Even a cheapie "quartz" watch has, at its heart, a synthetic quartz crystal. Lasers based on synthetic crystals are used in medicine in a wide variety of ways from surgery to removing tattoos to improving vision.
[Magnified picture of synthetic diamond crystals used as abrasives: Image courtesy of Dr. Jill Banfield] Beyond these practical applications, laboratory study and production of synthetic mineral crystals allows scientists to test hypotheses, and extend knowledge in many areas of the physical sciences.
**Check the web: This company produces a variety of synthetic mineral crystals for laser and optical use: http://www.enlight-tech.com/crystals-YAG.shtml
1) Melt (a molten material solidifies)
2) Solution (a solid is precipitated from a liquid in which it was dissolved)
3) Vapor (a solid material condenses from a gas in which it was dissolved)
For each of these we can think of everyday examples: if you have ever made hard candy, you will be familiar with the melt/solidification process, the calcium deposits on faucets and the water spots on dishes are examples of crystals derived from a solution, and frost on the window pane is a commonplace example of the vapor mechanism.
In this class we will survey the most widely used gem crystallization processes, and the types of gems they produce:
Melt Processes:
1) Flame Fusion
2) Czochralski "Pulling"
3) "Skull" Melting
Solution Processes:
1) Hydrothermal
2) Flux
Vapor Process:
1) CVD
In each of the melt processes the powdered solid ingredients necessary to make the gem are brought to their melting point and then allowed to cool in such a way that a single large crystal or cluster of large crystals is formed. The three processes, each suitable for making different materials, differ primarily in the temperatures used, the type of container, the heat source and the nature of the surface on which crystallization occurs.
1) Flame Fusion: The process commercialized so successfully by Verneuil, is termed "flame fusion". It is simple in theory and in practice. Corundum is Al2O3, crystallized in the trigonal system. All that is necessary is to melt the raw material, aluminum oxide powder, and allow it to crystallize. If you want ruby, you just need to add a small amount of chromium oxide to the mix (Cr is the chromophore that creates red in ruby). Although the process has been scaled up for today's large factories, it is, in essence. unchanged since Verneuil's time. Synthetics produced in this manner are the least expensive, and most commonly used types. So much so, that over one billion carats per year of flame fusion synthetic corundum, synthetic star corundum, and synthetic spinel are made.
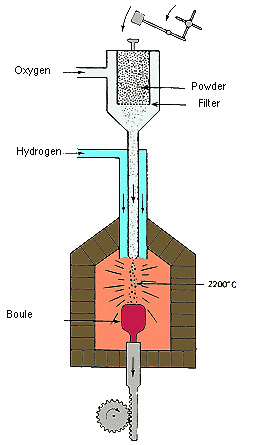
[The Verneuil "flame fusion" process for production of synthetic gems] The powdered ingredients fall into a chamber heated to 2200 degrees Centigrade by an oxygen-hydrogen torch flame. As they fall they melt. Upon reaching a ceramic rod in the bottom, cooler, area of the chamber, they crystallize. Slowly the ceramic rod is turned and lowered creating from the melted corundum a carrot shaped crystal called a "boule". The shape of the boule is characteristic, and is not one that is found in Nature.
This unnatural curved shape results in severe strain in the crystal lattice which necessitates splitting the boule in half before it can be cut. This somewhat limits the size of synthetic gemstones. It is possible (at considerable extra expense) to use a special slow cooling regimen, (similar to that used in glass blowing factories) to reduce strain, so that whole boules can be cut.
The Verneuil process takes about 4 hours and results in boules, at maximum, of about 20 x 70 mm (3/4" x 2 3/4"), weighing approximately 4-500 cts. Various elements can be added to create different colors (Ti and Fe for blue, for example) and rutile (titanium dioxide), can be added to create star stones. A special heating and cooling regimen is necessary for the star stones in order to force the rutile to exsolve into needles, rather than remaining dissolved.

[FF synthetic ruby: split boule, faceted stone, star stone] Food for thought:
Question 1: Think back to what you learned in Lesson 7 about faceting: Will a 20 mm wide split boule yield a 20 mm round brilliant?
Flame fusion synthetics are among the easiest types to identify. Their most characteristic feature is curved growth rings (called curved striae) that can be seen under magnification with appropriate lighting. In addition, sometimes coloring is uneven and follows the growth, making for curved color zoning. In the single crystal gems made by Nature, there are never any curved growth features or curved color zoning, so their presence is a dead give away. The presence of bubbles is also occasionally a tell-tale sign.
[Curved striae seen in a cut synthetic ruby at 25X, under diffused lighting] Wouldn't you know it? It is sad but true that, as consumers and marketplace watchdogs become more knowledgeable and sophisticated about gems, the deceivers get trickier. This is the case with flame fusion synthetics which are being passed off as natural--> by reheating the cut gems to near their melting point the growth layers can be partially fused with the result that the appearance of the curved striae is diminished, making these pieces harder to identify.
2) Czochralski "Pulling": It was the needs of science and industry, rather than those of the jewelry trade, which prompted the development of the Czochralski "pulling" melt process. Emerging laser technology demanded larger diameter, strain-free, higher clarity crystals than could be produced by the flame fusion process. Only later did the jewelry market begin to eagerly absorb some of the production.
In this process, the gem source materials are melted in a metallic (usually platinum) crucible using radio frequency energy. A thin, flat, seed crystal (either natural or synthetic) is lowered to just touch the surface of the melt, then slowly rotated and withdrawn ("pulled"). The slower the rate of pulling, the larger the diameter of the resulting crystal boule.
The seed gives the solidifying materials both a surface on which to crystallize, and an an atomic scale "pattern" to follow. This seed area is generally removed before the rough is sold.
Although many exotic materials (like gallium arsenide) are made for exclusively for industry, YAG, corundum, Alexandrite, and cat'seye Alexandrite are the major gem materials produced by this method. Production expenses are much higher than those of the simple flame fusion process, so the products are more costly as well.
[Green YAG: a simulant for emerald and Tsavorite garnet, and a durable, brilliant material in its own right] Pulled synthetics are very difficult to identify microscopically as the crystal that forms on the seed is usually flawless, and the large diameter of the boule makes observing the curved striae difficult. On occasion, they may contain triangular or hexagonal platinum crystals eroded from the walls of the crucible, which will conclusively identify the piece as synthetic.
[The very subtly curved striae seen on a magnified photo (25X) of the pear shaped green YAG shown above, would be easy to mis-identify as straight, the triangular platinum crystals in this magnified synthetic Alexandrite are a dead giveaway, though: Image courtesy of Martin Fuller] Food for thought:
Question 2: Why should larger diameter boules make for less obviously curved striae?
Question 3: If you were an honest seller of synthetic ruby jewelry why might you pick flame fusion material, and if you in the same business dishonestly, why might your choice be pulled synthetics instead?
3) "Skull" Melting: The commercial production of cubic zirconia, first accomplished by Russian scientists in the 1970s, required some ingenuity. The melting point of CZ is well over 2300 degrees C, which rules out the use of metal or ceramic crucibles. The problem was solved by using an externally cooled crucible filled with the powdered ingredients and then heating it with focused radio energy that melted only the center. The unmelted material formed its own insulation, or "skull". As the melt slowly cooled, large, usually flawless, crystals were formed.
**Check the text: see page 327 in the Lyman book for a figure representing the basics of the skull melting set up.
At present, CZ is the only material produced in this way, and costs are kept down by the large yields from each batch (currently the retail price of CZ rough is about $.05 per carat). Various colors are produced, but for the great majority of CZ is sold in its colorless form as a diamond simulant.
Although it is easy to identify by its optical and physical properties like density, thermal conductivity, and dispersion, microscopically, there are few signs of CZ's synthetic origin. Rarely, tell-tale bubbles can be seen. (I had to examine quite a few CZ pieces before finding this one lone bubble to photograph).
[Colorless CZ, today's diamond simulant of choice, few inclusions or growth features, other than an occasional bubble, give clue to the synthetic origin of CZ]
Solution Methods:
The characteristic feature of these processes is that rather than being melted, the source materials are dissolved in a solvent (not always water), and put under high temperature and pressure. The supersaturated solution is slowly cooled, and the gem crystallizes onto a natural or synthetic crystal "seed". If the solvent is water, the process is termed "hydrothermal", if it is another substance, then the process is called "flux". Because of the high temperatures and pressures involved, strong sealed metal containers are used to hold the solutions.
Some gems, like emerald and quartz, can only be made by solution methods. In other cases, such as with ruby and sapphire, solution methods are an alternative method of production.
Perhaps some of you remember crystal-forming demonstrations in junior high school science class. If not, the basics of this idea can be summarized by thinking of the formation of "rock candy".

This old fashioned treat is simply very large crystals of table sugar (sucrose) on a string. It is made by dissolving sugar in hot water until the solution is supersaturated (more sugar than will dissolve at room temperature). A wet, rough surfaced string, is rolled in dry table sugar, then hung in the solution, which cools and from which the water slowly evaporates. The dissolved sugar then crystallizes on the string. (If no string is present, the sugar will simply crystallize as a mass on the bottom and sides of the container.)
**Check the web: Here is a web site with a step-by-step recipe and directions for making rock candy--> this might be a fun family project: http://www.michigan.gov/hal/0,1607,7-160-15481_19268_20778-52395--,00.html
Food for thought:
Question 4: What is the purpose of rolling the string in the dry table sugar, wouldn't the string itself provide a surface for crystallization?
1) Hydrothermal: As the name indicates, this type of process uses water as the solvent. The vessel in which the gems form is lined with silver and referred to as a "bomb". (One can only speculate as to how it got this nickname, however, if you've ever misused a pressure cooker in your kitchen, you might have an idea!).
Hydrothermal synthetics are relatively expensive, as the equipment used is pricey, and the yields are small and slow to form (weeks to months). Because this process so closely mimics what occurs naturally in the Earth's crust, the majority of inclusions in such gems are natural looking, making them hard to identify. Occasionally, cut gems will show part of the seed plate or a distinctive non-natural looking inclusion called a "nailhead spicule". The primary gems produced by this method are emeralds, corundum (especially ruby) and quartzes in a variety of colors including blue.
[Hydrothermal synthetics: emeralds: Image courtesy of www.thaigem.com, rubies: Image courtesy of Tairus Corp. and a large chuck of colorless quartz: Image courtesy of www.faceters.com] 2) Flux: In some cases the solvent in which the gem source materials are dissolved is not water, but another material like lead fluoride or boron oxide, called a . These materials have in common that they have a lower temperature of cystallization than the gem. As the temperature in the crucible is lowered, then, the gem crystallizes first, separating out of the still-liquid flux. The process is slow and expensive. The highly corrosive nature of the fluxes means that crucibles must be made of a very resistant metal like platinum, iridium or gold, and the process can take months.
Ruby, sapphire, quartz, emerald, Alexandrite, YAG and red spinel are the major gems produced in this way. Although expensive as synthetics go, the resultant gems have natural looking inclusions, the most notable of which are sometimes called "wispy veils". Consisting of crystalized flux within minute cracks in the gem, they are so like natural fingerprint inclusions in their appearance that it takes a well-trained eye to discriminate them.
S
[Flux rubies, magnified "wispy veil" inclusions in a flux grown synthetic ruby Images courtesy of www.thaigem.com ]
Vapor Method:The third possible way to make gems synthetically is by vapor deposition. At present only diamonds (see below) have been made this way.
*(In discussing the following types of man-made materials, I will be putting the term, synthetic, in quotes. Although these gem substitutes are chemically and physically as close to the real thing as it is currently possible to make them, they still lack some of the crucial attributes of their natural counterparts. To strictly observe the niceties of gemological jargon, I should call them simulants, but as they are sold and generally discussed as synthetics, I'll simply use the quotes.)
Into this category go "synthetic" corals, turquoise, and opals all of which, (compared to the true synthetics above) are relatively easy to separate from natural. Under magnification each of them reveals its man-made characteristics: the coral lacks the characteristic biological ultrastructure of natural coral, the turquoise has a slightly bumpy surface, reminiscent of Cream of Wheat cereal that is most unlike that of natural stones, and the opal, shows an unnatural and distinctive "chicken-wire" hexagonal structure that gives it away.



The first successful synthesis of diamond was accomplished in 1955 by scientists at General Electric Corporation. These were tiny, .15 mm. crystals meant for industrial use. By 1958 the cost of synthetic diamond "bort" was competitive with natural, and today industrially produced diamond abrasives dominate the market. Besides their obvious superiority as abrasives, industrial diamonds find other applications as heat sinks and corrosion protectors. GE is still a major player, but shares the world market for industrial diamonds with Sumitomo and DeBeers Corporations.
HTHP: General Electric was also the first to produce gem synthetic diamonds in 1970. These were small and quite yellow. The nitrogen in atmospheric air incorporates into the diamond during the formation process, and acts as a chromophore giving the resulting crystal a yellow color. GE's method, in essence, attempts to replicate the conditions deep within the Earth's mantle where diamonds form naturally. Called HTHP (high temperature, high pressure), this, still somewhat secret process, involves a metal solvent/catalyst, 60,000 atmospheres of pressure, and temperatures of at least 1500 degrees C. The machines in which the diamonds are formed are called "presses". For the last thirty years or so, synthetic gem quality diamonds have been largely a laboratory curiosity as they cost more to produce than it would cost to obtain equivalent stones naturally.
All this is about to change! Several companies are now involved in producing, or gearing up to produce, gem synthetic diamonds for the jewelry market. Gemesis and Chatham are two manufacturers of note-->Gemesis is already in the retail sales mode, specializing in intense yellow colors with gems nearing 2 cts, while Chatham is poised to enter the market shortly with pinks and blues as well as yellows.
To date, the high expenses and difficulties in attempting to keep the yellow color out, have given little motivation for the HTHP manufacturers to try to compete in the colorless diamond market. Fancy color diamonds are a much more profitable product. It remains to be seen if the public will be willing to pay the, still very high, prices (about 30 - 40% the price of natural fancy color stones) for synthetics.
[Gemesis created fancy color diamonds: Image courtesy of Gemesis Corporation, Chatham created fancy color diamonds: Image courtesy of Tom Chatham] [Diamond "Presses" engaged in producing synthetic diamond by the HTHP method: Image courtesy of Gemesis Corporation] CVD, Chemical Vapor Deposition: The third of the possible gem synthesis processes (condensation from a vapor) has been pioneered, by Apollo Corporation, for the production of diamonds. In this approach, a vacuum chamber (at .1 atm of pressure) containing a thin diamond seed crystal is filled with methane gas (CH4) at 1000 degrees C. At that temperature and pressure regime, the carbon in the gas separates from the hydrogen, and crystallizes upon the diamond seed surface.
Early on, the resulting crystals, which were wafer thin, were intended for industrial applications, primarily as the future generation of heat resistant computer chips. As the methodology has improved, thicker and thicker crystal wafers have been formed. At present, Apollo gem quality diamonds of .25 ct have been cut. Compared to HTHP diamonds, they are relatively white and have high clarity. It looks as if it won't be long until gem synthetic diamonds are a profitable side-line for this company.
[Vacuum chamber used by Apollo Corporation for CVD diamond synthesis: Image courtesy of Apollo Corporation] [The first batch of cut "gem" synthetic diamonds produced by CVD: Image courtesy of Apollo Corporation] **Check the web: Apollo's novel approach, and good looking results have gotten several rounds of media coverage since the first gem diamonds were submitted in August, 2003 to GIA for analysis: Here's an early example from USA Today: http://www.usatoday.com/money/industries/technology/2005-10-06-man-made-diamonds_x.htm What does the diamond world think of all this?? One of the hottest topics of discussion in gemological and jewelry marketing circles these days, is synthetic diamonds and their potential impact on the natural diamond market. Although some analysts have been quick to cry doomsday, and predict the fall of the natural diamond industry, the majority opinion is that created diamonds were inevitable, and having arrived, should be viewed as a positive development. Enhanced and man-made diamonds are expected to share the market with natural origin/natural color stones, as have synthetic and enhanced rubies, sapphires and emeralds with their natural counterparts.
The major concerns within the industry have been those of 1) disclosure 2) identification and 3) cost.
1) Disclosure: At present, the major companies producing gem synthetic diamonds have taken pains to identify their products as synthetic, and are selling them only through reputable and licensed jewelers. Gemesis for example, puts a laser inscription on the girdle of all its .25 ct or larger gems. (Unfortunately, it is a relatively simple matter to remove this ID after the gem leaves the store, and there is evidence that this is already happening).

2) Identification: The conclusion that the market is large enough for both synthetics and natural stones is based on the assumption that the two can be reliably discriminated. Although HTHP gems have tell-tale features that enable a well trained and well equipped gemologist or jeweler to identify them, CVD diamond recognition currently is possible only for major laboratories. The statement below was issued as part of official press release by GIA.
"The major laboratories can conclusively identify gem synthetic diamonds" William Boyagian, President of GIA, 9/19/03 3) Costs: Unlike diamond simulants which can be detected with a simple, inexpensive electrical conductivity tester, synthetic diamonds, are diamonds and therefore pass all the standard physical and optical tests, as diamond.
DeBeers Corporation (the company that controls the majority of worldwide diamond rough sales) has been in the forefront of synthetics detection, and offers two table model devices (costing over $20,000) that can identify HTHP stones. The DiamondSureTM which tests for a specific 415 nm absorption line, and gives a reading of either: "natural" or "refer", and the DiamondViewTM which detects characteristic strain patterns and can conclusively identify the HTHP synthetics amongst the "referrals".
[Licensed for sale through GIA-UK, this "DiamondView"TM instrument can be used to conclusively identify HTHP diamonds: Image courtesy of GIA-UK] CVD diamonds cannot be reliably detected even with these instruments. The average neighborhood jeweler is understandably concerned about the costs that will inevitably ensue as verification of natural origin in diamonds becomes the sole pervue of the big labs. (Testing with Raman Laser Spectroscopy and Fourier Transform Infrared Spectroscopy at the temperatures of liquid nitrogen, doesn't run cheap).
To grade or not to grade, that is the question... Very heated arguments can be started among those involved in the diamond business over whether synthetic diamonds should be graded (for color and clarity) or not. GIA, AGTA and most other big gem labs have taken the position that they will identify synthetics, but will not grade them. It is their assertion that synthetics, have no inherent value, and, as such, should not be graded. EGL (European Gem Labs) and its American subsidiary, EGL-USA are grading the synthetic stones for clarity and color. Their philosophy is that customers want their stones graded, and they are providing a desired service.
Before discussing cultured pearls, let's take a brief look at natural pearls. Natural pearls are very rare today, but they were always rare. The product of a mollusk's reaction to an irritant, such as a stray bit of shell or a parasite, they take many years to grow to useable size, and few individuals within a population of mollusks will ever make them. Beyond that, the majority of pearls in Nature are small, baroque in shape, and few are blemish free. Throughout most of history, natural pearls were the rarest and most highly valued of all gemstones.
Here are some examples of natural pearls and how they were used. As the vast majority of pearls that were collected were tiny, those who could afford pearls, but didn't have the budget of Cleopatra or a Roman Emperor, often used seed pearls (2 mm or under). Matched strands of larger round pearls, though quite expensive, were very small by today's standards, and not perfectly uniform in either size or color.


**Check the text: The picture on page 278 of the Lyman book will give you a good idea of the color and size variability of natural pearls and the pictures on page 281 will show their typical baroque shapes.
At the turn of the 20th century, there were several individuals experimenting with culturing pearls, and in 1906 the first batch was grown. It remained, however; for Kokichi Mikimoto to both scale up the process to mass production, and market the products successfully to an initially skeptical world. By the end of the 1920's cultured pearls were an accepted part of the pearl marketplace, and today they are the marketplace. Except for those found in some antique and vintage jewelry items, and a few natural pearls sold to collectors, all the pearls in commerce are cultured. "Cultured" was a term that was hotly debated initially, with many feeling that the term "synthetic" should be used to clearly specify their non-natural origin, but Mr. Mikimoto won that fight. (Regardless of terminology, though, cultured pearls are not natural gems, their origin is the result of human intervention.).
There are two basic types of pearls (be they cultured or natural). A pearl that forms within the body cavity of the mollusk, and has a three dimensional shape, sometimes round, is a "cyst" pearl. One that forms attached to the shell of the animal, and therefore has a flat back surface is known as a "blister" pearl. Possible colors of pearls vary with the species of mollusk, but usually are some shade of the color of the shell lining of the animal.
An assembled, cultured, blister pearl product is known as a "Mabe" pearl. They are grown by gluing a plastic or shell half dome or other shape (nucleus) onto the inside surface of a mollusk's shell. Once the nucleus is coated with nacre, the blister pearl is cut away from the surrounding shell. The nucleus is removed and the cavity is filled with an epoxy resin, and backed by mother-of-pearl. Mabe pearls are quite attractive, less expensive alternatives to cultured pearls of the same diameter.



Natural pearls come from mollusks native to both saltwater (oysters) and freshwater (mussels), and cultured pearls can be produced from both types of mollusks. Two different strategies are used in culturing: bead and tissue nucleation.
Saltwater: Saltwater cultured pearls are "bead nucleated", meaning that a round bead of shell is used as the "irritant" around which the oyster secretes the nacre. The company that Mikimoto founded is still going strong, and specializes in the highest quality saltwater cultured pearls from the Akoya oyster. Such pearls are white to silver or cream, often with pink overtones. They tend to top out at about 8 mm in size, and those pieces with relatively thick nacre layers due to long cultivation periods, have a fine luster.
Larger pearls, such as Tahitian blacks and Indonesian golds, are cultured in oyster species from the tropics, and can attain sizes of 20 mm or greater. Saltwater cultured pearls can be rather expensive depending on size and quality.


In the picture below an oyster is being nucleated with a shell bead. You can see the animal in the holder, and the dish of beads. Expert nucleators command respect within the industry as skilled professionals, and are very highly paid.

Freshwater: Freshwater cultured pearls are almost always "tissue nucleated" which means that only a slice of mantle tissue from a donor mussel is used to start the culturing process, there is no shell bead. As is the case with natural pearls, a tissue nucleated freshwater pearl is virtually all nacre. Historically, freshwater pearls were first produced from Lake Biwa in Japan, but today most come from China. The original "Biwa" pearls, introduced in the 1970's were small with flat, baroque shapes--> they were sometimes referred to, dismissively, as "rice crispies".
You can see in the second photo below that things have changed, and today's freshwater cultured pearls are large and approach a perfectly round shape. Freshwater pearls remain inexpensive: the strand at the left retailed when new (1980's) for about $30 and the 18", 7.5 mm strand to the right currently retails for under $200.

Many saltwater cultured pearls can be identified by microscopically viewing the drill hole and observing the demarcation between the shell nucleus, and the nacre layer on top of it. In difficult cases, and with freshwater cultured pearls, Xrays will do the job. The bead nucleus shows up clearly in the radiographs, as does the smaller area where the mantle tissue was placed in a freshwater cultured pearl. There is actually little chance, though, that today's fresh or saltwater cultured pearls would be mistaken for natural pearls. To anyone who has studied natural pearls, today's creations are so large, so round, and so well-matched in color, that the difference is obvious. Making the call between enhanced and unenhanced is another matter though. As you recall from Lesson 8, most cultured pearls are enhanced in one way or another.
Also known as imitation or faux gems, simulants look like what they imitate, but they don't have the chemical, physical and optical properties of the gem they mimic. (**Just a reminder from Lesson 1, simulants are only fakes if they are not properly disclosed.) Some are man-made, and some are natural gems in their own right.
Early Simulants The history of gem simulation goes back every bit as far as that of gem enhancement. Since before 3000 years ago Egyptians have been making faience, a beautiful blue to green non-clay ceramic material. The colors are derived from copper or other metals and, although used and admired for its own sake, it has also commonly been used to imitate turquoise and lapis lazuli.
[Faience "mummy" beads, circa 300 BCE (newly re-strung to wear), 1880's Victorian "Egyptian Revival" brooch featuring "plique a jour" enamel and an ancient faience scarab] The technology required to make faience is similar to that necessary to make glass, which the Egyptians also mastered. Glass making skills are believed to have traveled to the Greco-Roman world from Egypt, and by 1000 BCE, the Romans began casting glass. (Glass blowing came along much later).
[A fragment of circa 500 CE Roman glass made by blowing molten glass into a mold (the iridescent surface patina was not a glaze or paint put on by the Romans, but has been slowly created by chemical reactions, between the glass and soil, occuring over centuries of burial, these reactions creates a thin layer of metal oxides) ] Simple glass beads and cabochons were in used in jewelry--> in fact they were enjoyed only by the wealthy, as these technological marvels of the day were more valuable than most natural gems!
[Circa 1000 CE Chinese glass bead (on modern chain), Roman bronze and glass cabochon ring circa 100 CE] Natural Gems as Simulants: It is very common for one natural gem to be used to imitate another of similar appearance. For example serpentine, aventurine quartz and hydrogrossular garnet, all have long histories as jade simulants. Likewise, bone is a common substitute for ivory, and white zircon has long been enjoyed as a natural diamond simulant. Red spinel commonly is substituted for ruby, sodalite for lapis lazuli, and copal for amber.




Glass: Although its historical roots go way back, glass is still one of the most popular gem simulants today. Glass, itself, is an amorphous material, but its main raw material, silica sand (quartz), is crystalline. In glass-making sand is mixed with certain other materials and melted; then it is cooled so quickly that crystallization doesn't occur. Some scientists describe glass as a liquid that is so stiff that it behaves, superficially, like a solid. This assertion is supported by the observation that very old glass windowpanes are thicker at the bottom than at the top, and the older they are, the more this effect is seen.
Two quite different forms of glass are used to simulate gems: crown glass and flint glass.
Crown Glass: is used for ordinary windows and bottles, and is also known as common, or silica glass. When gems are made from it, it is often called "paste". This sort of glass is relatively soft, has a low refractive index, a low specific gravity, and virtually no dispersion. Due to its lack of brilliance (a consequence of the low refractive index), a metallic foil or paint was often applied to the back facets of such gems (called "foilbacks") to increase reflectivity.
Flint Glass: is also known as "crystal", "strass" (named after the 18th century inventor), or "leaded glass". This very soft, easily scratched glass has a high refractive index, high specific gravity, and high dispersion.
** Check the web: Just about the most famous producer of gem "crystal" (highly dispersive leaded glass) is the Swarovski company, visit their site at: http://www.swarovski.com/
The natural color of glass is a light green (perhaps you have seen or remember the color of the original "Coke" bottles), but it can be de-colorized or colorized chemically. By adding different colorants (chromophores), spectacular hues can be achieved, like purple (manganese), dark blue (cobalt), and red (gold).
Identifying glass: Many paste gems are molded rather than faceted, and as such, have notable surface characteristics, such as sunken facets, an orange peel surface, or mold lines. Even if glass is faceted, its softness often results in facet edges that are slightly, to noticeably, rounded compared to the knife-edge facets of harder gems.
Physical and optical characteristics are also useful in identifying glass. The typical bubbles and swirls, seen under magnification, have already been mentioned in Lesson 5. Added to this are the low hardness (4-6, depending on the type of glass), the characteristic brittleness, and the conchoidal fracture with its vitreous luster. Furthermore, the relatively low thermal conductivity of glass makes it feel somewhat warmer in the hand than crystalline materials. As an amorphous substance, glass will give a polariscope reaction of SR, and in terms of density the specific gravity of crown glass is less than most common gems, while the specific gravity of flint glass is higher.
Historically, glass has been used to imitate just about every kind of gem. Today, however, glass is most commonly used to simulate translucent gems such as opal and chalcedony, and opaque gems like jet, lapis and coral. (Over the last 100 years or so, the availability of synthetic gems has dramatically decreased the popularity of glass "rubies" and other faux transparent gems). Even phenomenal gems such as moonstone, sunstone and pearls have glass simulants. Faux pearls had their heyday of popularity before the advent of pearl culturing, but many types are still made today. Commonly they are made of translucent glass beads that get a many-layered coating of lacquer containing pearly looking "essence d' orient" (made from ground fish scales!).
The moral of the story for the gemologist is: "Always suspect that the "gem" you are looking at is glass--> and test for this possibility."






Plastic: Most people would be surprised to learn how far back the manufacture of plastics goes. By the late 1800's the "plastic age" was well into its beginnings. The earliest plastics, like vulcanite, Bakelite, celluloid and lucite were used for a wide variety of purposes, including among them gem simulation. Some of these early materials found use as imitations of jet, ivory and tortoise shell, making copies of these luxury organic gems available to a large audience. Plastic jewelry peaked in popularity in the 1950's, but still finds use today, mainly in the cheapest of simulants (the sort you might get from a gumball machine, or as a prize at a carnival).
Visually, plastics can be recognized by the greasy to sub-vitreous luster, rounded facet edges, surface craters or pits, and the presence of mold marks. The surface of its fracture has a dull luster. Microscopically, plastics have swirls and bubbles similar to those seen in glass. They give an SR reaction to a polariscope test.
Plastics are quite soft, with hardnesses ranging from 1.5 to 3, and both the refractive index and specific gravity are low. Even more so than with glass, the low thermal conductivity of plastics makes them warm to the touch. When touched to the surface of plastic, a "hot point" tester will release an acrid chemical smell that is quite distinctive.
(Here's a method of identification that I wouldn't recommend with a prize antique: drop the piece from about 5 inches onto a hard surface like a desk top. Plastic makes a hollow sound compared to the higher pitched, sharper sound made by either glass or crystalline gems.)
Glass is a more convincing simulant of transparent gems, but plastic does a good job imitating translucent and opaque stones like amber, turquoise and coral. (A sizable percentage of the least expensive Southwestern sytle "turquoise" jewelry is actually plastic.) Certain optical properties of some types of plastic can be utilized do a good job of simulating phenomenal gems like opal and moonstone.






The bad news in regards to diamond simulants is that there are a multitude of them, no other gem has been so often imitated. The good news is that all of these mimics can be discriminated by either simple observation, or by basic gemological testing-->no big labs needed!
The oldest diamond simulants were glass, and colorless natural gems. With glass, first came the older crown type (usually with a foiled back to increase reflectivity), and later the more brilliant and dispersive flint glass. The natural gems white topaz, sapphire, zircon and quartz were also used, but at a higher cost than glass.
(If you are skeptical that glass or quartz could make a reasonable approximation of a diamond, consider that with the simple early diamond cutting styles, and the polishing technologies available prior to the 20th century, diamonds were not the blindingly brilliant gems of today.)
Synthetic diamond simulants enter the picture around 1910 when colorless sapphire was first produced by the flame fusion process. This was followed by the introduction of colorless synthetic spinel (1920), synthetic rutile (1948), strontium titanate (1955), YAG (1960), CZ (1976), and Moissanite (1996).






Glass: Crown glass is notably softer, less dense, less brilliant, and less dispersive than diamond. Flint glass though dispersive, is more dense, and much softer. Both types have a vitreous luster, as opposed to the adamantine luster of diamond.
Natural Gems: Depending on the species, the RI, the density, or the dispersion would be the give away, and all the commonly used colorless natural gems are DR, so a polariscope test would easily separate them from the SR diamond. Zircon, the closest to diamond in appearance is not only DR, but so strongly birefringent that it shows doubled rear facet images when viewed with a loupe.
Synthetic Sapphire/Synthetic Spinel: Although hard, corundum is a DR gem and its RI of 1.76 (diamond's is OTL), and lack of dispersion is telling. As spinel is an SR gem a polariscope test wouldn't help, but its RI of 1.72, hardness (8), and low dispersion would be the best indicators.
Synthetic Rutile/Strontium Titanate: These gems enjoyed a period of brief popularity after their introductions, and are sometimes seen in vintage pieces. Indentification is a simple matter though, as their extremely high dispersion makes them very easy to spot, and their softness makes them noticeably fragile compared to diamond.
YAG: Although hard (8.5) and SR, YAG is so lacking in dispersion that it, too, can be easily separated from diamond.
CZ: The remarkable popularity of CZ is, in my opinion, well deserved: hard (9), SR, and not too noticeably more dispersive than diamond, it passes most visual tests well, but testing for density will reveal it every time, as it is much heavier than diamond.
(In addition, all of the simulants above will "fail" a thermal conductivity test.) Moissanite: This is the latest contender, and like CZ, it is hard (9.5), and not so overly dispersive as to be obvious. It will pass a thermal conductivity test as diamond, but its electrical conductivity will invariably identify it. In light of this, a new generation of "diamond testers" is being highly promoted as indispensable to those concerned with guarding against bogus "diamonds".
Such equipment not really necessary though, as Moissanite is DR, and will flash "on and off" with the polariscope test described in Lesson 4. Even without fancy gemological equipment, all that is needed is a loupe, and a little knowledge. Moissanite gems are deliberately cut on an optic axis so that when you look down through the table you see sharp facet reflections (no doubling) as if the stone were SR. By tilting the gem on its side, and viewing the back facets with a loupe, the strong birefringence of this DR gem will reveal itself by the presence of doubled facet images, something you'd never see in a diamond-->no matter which direction you turned it.
[Magnified view of doubled facet reflections in Moissanite]
Also known as composite gems, assembled gems are made of two or more pieces of gem material joined together. They can be used as to simulate (or fake) another gem, or just for their own sake, for example to make an artistic gem creation, or to make a particular gem material more durable or useable.
Assembled Gem Art Intarsias, insets, inlays, pietra dura and micro-mosaics fall into the category of making something beautiful out of what would otherwise have been waste products. These lovely works of gem art are often made of the small bits and pieces left over from, or too small for, other lapidary activities.







Doublets and triplets are generally made either to increase the durability of a fragile gem or to support and protect a thin slice of gem material. Usually a doublet consists of a thin layer of some valuable, fragile, or rare gem material backed with a thicker layer of something sturdy, and less expensive. If the piece is unmounted, or set in prongs, it is usually very easy to see the demarcation line between the top and the bottom. Sometimes the backing is simply used to provide contrast or create an artistic effect. A triplet is a doublet with a third, top, layer made of a tough transparent material like colorless quartz.




Historically, assembled gems were more important as gem simulants and fakes in the days before synthetics became widely and inexpensively available. You are more likely to see them in vintage and antique jewelry pieces., but there are still a few cases, where assembled stones are used as gem stand-ins, and some unscrupulous folks are still trying to pass some types off as something they aren't.
Not for Deception Mabe pearls, as described in the cultured pearl section above, are examples of assembled gems which are produced with no intent to deceive. They always have flat backs and mother of pearl bases which easily distinguish them from regular cultured pearls.
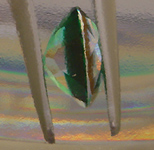
[Black Tahitian Mabe pearl earrings] The green stone below is a simulated "Soude" emerald such as is regularly used in the marketplace today. Usually, at least at the retail level, there is no intent to deceive, and these are sold widely and openly as an imitation May birthstone (emerald).
A Soude is actually a three piece assembled gem--> a triplet. The top and bottom layers are made of colorless synthetic spinel created by the inexpensive flame fusion process. The middle layer, depending on the manufacturer is either green glass or, as in the stone below, green glue. Because the stone is faceted, when viewed from the top with light reflecting throughout its interior, selective absorption from the green layer makes the stone look uniformly green.
It is not uniformly green, though, as you can see in the second photo, where the stone has been immersed in liquid and is viewed from the side. The colorless top and bottom are clearly visible. Once the stone is set however, such a test is difficult to do.
Another "tell" for an unmounted or prong mounted piece of this type, is the magnified view of the girdle area. As the third photo below shows, the "join" or seam between the crown and pavilion, is visible. In a single-piece gem, no such line would be there, the girdle area would be unbroken.


The classic case of deception with an assembled stone is the "garnet and glass doublet" which was used throughout the 19th and early 20th centuries to mimic various colored transparent gems. In these clever concoctions, the bottom and most of the top of a piece of "rough" is constructed from colored glass to which a thin slice of natural (red) garnet has been fused or glued. The rough is then faceted so that the thin sliver of garnet forms some of all of the crown area of the gem. The color of the glass pavilion is all that shows face up, especially when the doublet is mounted. The garnet top provides high luster and good durability that the glass bottom lacks.
By microscopic examination, or immersion and viewing from the side, the deception can be seen, but once mounted, these frauds almost always escape detection.
** Check the text: The Hall book has pictures and information on classic G&G doublets on both page 37 and page 61. (She calls them "garnet-topped doublets")
In today's marketplace there are still a few doublets and triplets being manufactured to fool the unwary, but due to the low cost and easy availability of synthetics, they are not as common as they once were. Some examples that jewelers and auction houses occasionally still see are YAG/strontium titanate doublets (as diamonds), synthetic ruby/ruby doublets (as ruby), and beryl triplets (as emerald).
Food for Thought:
Question 5: Why would a YAG and strontium titanate doublet be a better diamond simulant than either material by itself?



Detecting assembled stones can be as easy as simply looking at them, as in the case of intarsias or inlays, but in other cases careful testing is necessary. The best bet for a gemologist, jeweler or collector is to always check the RI of both the crown and pavilion of the stone, and observe both the crown and girdle area under magnification and under immersion if possible. Prong mounted gems can usually be examined adequately as is, but bezel set stones (especially those with completely closed backs) often must be dismounted if their identity is to be conclusively verified.
Answers to the thought exercises for this lesson. (If you don't understand something in these answers it's time to email me and ask!)
1): A cylindrical boule that is split down the center will have a depth of 10 mm, so theoretically the maximize sized round stone you could get would be 10 mm. In practice it would have to be even less than that in order to insure there was no window.
2): Think of cutting a cherry in half and compare the rate of curvature of the outer edge with an orange cut in half. The larger the circumference the more gradual is the curve. When you are looking at a faceted gem you are looking at a tiny little sector of a boule--> the larger the boule it was cut from, the less curvature you will see.
3): An honest synthetic ruby seller will go for the lower priced flame fusion material whereas the dishonest one might opt for the more expensive, but harder to identify, pulled material.
4): The tiny sugar crystals not only provide a surface, they also provide a pattern to follow so that as the sugar in solution crystallizes, the ones that are already present get larger. Without this pattern to follow the sugar crystallizes in a more disorganized manner that is not so attractive.
5): A doublet made with YAG on top and strontium titanate on the bottom would be relatively hard (YAG is 8.5) and have less dispersion than strontium titanate alone (which has too much) and more dispersion than YAG (which has too little).
You have now completed the web lecture for the ninth lesson!
Go back the the course web site to: 1) complete and submit the homework assignment on the text readings and assigned web essays 2) take the non-graded practice quiz on this web lecture 3) post a comment to the discussion board for this lesson, and 4) when it is available, complete the graded quiz based on this web lecture.
When you're ready, proceed on to Lesson Ten: Gem Formation








.striae.YAG.jpg)