
|
|
The Metal-Planet Affinities - The Sevenfold Pattern By Nick Kollerstrom Back to Metal-Planet Affinities index page From antiquity up until the mid-eighteenth century, the number of metals known and recognised as such was seven. They were: lead, tin, iron, gold, copper, mercury and silver. Brass, made from copper, was used, but people didn’t realize it was an alloy that included zinc, until the latter half of the eighteenth century. The metal which finally broke the sevenfold spell of millennia (in 1752) and was called the ‘eighth metal’ was platinum, emerging from the gold mines of Columbia. Belief in a linkage of these seven metals with the 'seven planets' reaches back into prehistory: there was no age in which silver was not associated with the Moon, nor gold with the Sun. These links defined the identities of the metals. Iron, used always for instruments of war, was associated with Mars, the soft, pliable metal copper was linked with Venus, and the chameleon metal mercury had the same name as its planet. Then, around the beginning of the 18th century these old, cosmic imaginations were swept away by the emerging science of chemistry. The characters of the metals were no longer explained in terms of their cosmic origins but instead in terms of an underlying atomic structure. New metals started to be discovered which made the old view appear limited. In the 20th century new lines of approach to this old subject were opened up through work done within the Anthroposophical movement founded by Rudolf Steiner, and we here draw especially from the works of Rudolf Hauschka (2) and Wilhelm Pelikan (3). They viewed the traditional seven metals as expressing most fully the seven planetary characters, in a way that the many other metals known today do not: ‘The seven fundamental metals represent something like the seven notes of a scale. As there exists a great variety of intermediate tones within the scale, so one can recognise intermediate tones between the metals’ (4). The extra-light metal lithium is used for hydrogen bombs, anti-depressant pills and bicycle axle grease. Thereby one may feel its ‘lightness of being,’ but that won’t quite give us a planetary affinity for it. Magnesium emits a brilliant light on burning, used for photo flashlights, so does this give it a solar affinity? It is used for ultra-light alloys in supersonic aircraft etc, and is the key metal used in chlorophyll, whereby solar energy is metabolised by plants. Wilhelm Pelikan suggested that it should be viewed as a sun-metal, and let’s view this as a possibilty. Physical Properties We experience metals as differing from non-metals by virtue of their lustre, their resonance, their malleability and conductivity - these are their key physical properties. Metals can be polished to shine (lustre), will produce tones when struck, ie they sound (resonance), when hammered they don't shatter, they can be beaten into shape, and will quickly become hot if one corner is heated. The traditional seven metals can be arranged in a scale, by these key physical properties. This turns out, remarkably, to be the same scale as an ordering of their associated planets, in terms of their speed of movement. The Table below expresses metallic conductivity both as thermal (conducting heat) and also as electrical, scaled for convenience to silver = 100 (5). The planets are ordered by something which one can experience quite directly, namely how fast they move across the sky - from the Moon as the fastest moving to Saturn as the slowest. This means using a geocentric perspective, as we see their mean angular speeds from the Earth, and gives the traditional ordering as used to be assigned to the planets in the old, Ptolemaic system - for almost two thousand years. This ordering was almost universally accepted, up until the time of Copernicus, and had the sphere of Mercury nearer the Earth than Venus (mathematically, this may be the case: ie, Mercury is more often nearer to us than Venus (6)). Metal Conductivity and Planetary Motion Mean Metal orbital Associated Motion with Thermal Electrical Planet deg/day Planet Conductivity Conductivity Moon 13.2 silver 100 100 Mercury 1.4 mercury - - Venus 1.2 copper 94 95 Sun 1.0 gold 74 72 Mars 0.5 iron 20 17 Jupiter 0.08 tin 16 13 Saturn 0.03 lead 8 8To quote the modern biochemist Dr Frank McGillion, ‘The orbital motion of the planet correlates in sequence with its corresponding metal's conductivity… The slower a planet moves, the less able its corresponding metal is to conduct electricity!’ (7). For the alchemists of old, metals all had these properties to different degrees. They didn't view them as separate elements, but accepted that they had these experiential properties in common. In addition, a metal had to be purifiable in a furnace, where it would melt but not burn. This is why they could never take zinc seriously as a metal, because it just burnt up on being heated. This criterion put them in a difficult position over mercury, as was generally recognised as metallic, though paradoxically so. This experiential definition limits us to what we’ll call ‘real' metals, whereas the modern definition of a metal is wholly abstract - in terms of atoms that are electron-donors - and includes substances that don't at all resemble these: for example, potassium is a waxy substance that bursts into flame upon mere contact with water. Nowadays, children even in elementary science lessons are given these quite abstract concepts, and are hardly allowed to experience the primary properties of the everyday metals. Here we concentrate on things that are elementary. Let's go through what we are here calling the key physical properties: Conductivity: copper is used for electrical wiring being a good conductor, as lead is used for fuses because it is such a poor conductor. Mercury is not included on this table being a liquid - conductivities of metals when liquid are much lower than when they are solid. Lustre (or reflectance): silver is the most perfectly reflecting metal of the seven and is therefore used for making mirrors. Mercury also has a very high lustre and is likewise used for such: these are the two mirror-metals. In antiquity, mirrors of copper or bronze were used. The other metals show an approximate gradation in lustre down to lead which has a very dull surface. Resonance: copper is much used in musical instruments because of its high resonance although silver instruments have the clearest, purest tones - 'silver bells', and this property again decreases down the scale to the dull sound lead makes on being struck. Malleability: Hauschka described how metals at the top of the list are highly malleable, but cannot be well cast, whereas those at the bottom can be cast but not forged. Gold he described as holding a balance position in that it could equally well be cast or forged. These scales show an increase in inner mobility from lead, the most inert, up to silver, which parallels the increasing angular speeds of the planets. Hauschka, who first described this, concluded memorably: 'We see then that planetary movement is metamorphosed into the properties of earthly metals' (8). II Chemical Activity . ..‘This isn’t just a date, it’s chemistry from the film, ‘Something about Mary’ Moon Mercury Venus Sun Mars Jupiter Saturn silver mercury copper gold iron tin lead 1 1 & 2 1 & 2 1 & 3 2 & 3 2 & 4 2 & 4Silver, which showed the highest conductivity and gave the purest sound, has only a single valency for all the links it forms with other elements. Like swans which remain monogamous and faithful to one partner all their life, the Moon-metal silver has only one arm of valence. In contrast, those which scored lowest on their physical properties, tin and lead, being least conductive etc, are most active and greedy in their ratios of combination. Reactivity: Some metals are inert, for example gold hardly combines at all, and these are called ‘noble' metals (platinum, silver); whereas tin and lead are reactive and will dissolve even in weak acids. We can put the classical metals in a sequence of their chemical activity, which is conveniently measured by what chemists call their ‘electrode potential.' This tells us how reactive their ions are in solution. Inactive metals as will not liberate hydrogen from an acid are called ‘electronegative', while the more active metals which will liberate hydrogen are ‘electropositive'. This gives a useful scale of chemical activity for metals, measured by the ‘standard electrode potential' of a solution at a given concentration. Let’s start (as McGillian here advocated) with the order of the planets going out from the Sun, and then the corresponding electrode potentials of the metals are:
Sun Mercury Venus Earth Mars Jupiter Saturn
gold mercury copper iron tin lead
-I.50 -0.79 -0.33 +0.44 +0.14 +0.13
Electronegative Electropositive
Thereby McGillian contrasted the more reactive, ‘electronegative' metals as linked to planets inside Earth's orbit with electropositive ions which correspond to those outside the Earth's orbit (10). Electrode potential is measured with respect to that of the earth, which indicates the relevance of the geocentric viewpoint here involved. He concluded, ‘The earth-centreduniverse of the alchemists is polarised into positive and negative. It is chemically yin and yang.' A more traditional ordering would have silver at the top of the list and Sun-metal gold in the middle, which is how Hauschka described it; which has to use the notion of ‘above the Sun' planets, Mars, Jupiter, and Saturn having electropositive metals, while vice versa for ‘below the Sun' planets, not a very modern concept! Silver's standard electrode potential is -0.8. Either way, the correlations are impressive. III Atomic Weights Each element has an ‘atomic weight', and the Periodic Table of Elements arranges them in sequence of these atomic weights. It starts with hydrogen having an atomic weight of one, then for example carbon is 12 and oxygen has 16. This ordering by atomic weights gives insight into the chemical properties of each element. When Mendeleev discovered the Periodic table, by arranging elements in this way, he was able to predict the chemical properties of several elements that had not yet been discovered, and his theory came to be accepted as these were confirmed. Mendeleev's Table has seven rows or ‘periods,' from the first row that just has the lightest elements, hydrogen and helium, down to the seventh which has the extra-heavy, radioactive elements such as uranium and plutonium. Vertically, it has seven or eight columns (the eightth and last column with the inert gases is usually given as the 0th column, the others being counted as 1-7): so, in a sense it has seven columns, too. What are called ‘group one' elements belong to its first column, and these are all univalent, such as sodium. Group two (the second column) are bivalent like calcium, group three are trivalent, eg aluminium. So, the number seven appears in this Table as rather dominant, as controlling the possibilities of what elements can exist. When Uranus was discovered in 1781, by William Herschel, this definitely kicked out the notion that there was something sevenfold about the heavens. Up until then, there had been seven spheres which could be seen to move across the sky. There still were such indeed, but an extra unseen one had been added. After his discovery, there was no longer anything sevenfold about the world! This dire state of things persisted for nearly a century, until chemistry professor Dmitri Mendeleev formulated his Periodic Table. A seven fold pattern then reappeared in matter, in the science of chemistry. Bearing this in mind, it may be of interest to look at the moment in time when this new synthesis was created: the afternoon of March the first, 1869. There were no less than six septile-aspects then present in the sky, between the planets. They were: MO-SA (1°), VE-JU (1° 10'), MO-UR (0° 10'), ME-NE (1° 40'), VE-NE (0° 30'), SA-UR (1°) (The septile is a celestial aspect formed by dividing the circle into seven parts. It gives the angle of slope of the Great Pyramid, 51 1/2°) The cosmos was in quite a sevenfold mode at that moment in time, when the new synthesis dawned upon Mendeleev. It was a classical eureka-type situation: he had cut out cards for each known element, was trying to arrange them by their atomic numbers on his living-room carpet, dozed off, and when he woke up, it came to him! What here concerns us is the notion that a sevenfold pattern is discerned in matter, during a period when these are quite strongly present in the heavens. In ordering of the classical seven metals by their atomic weights derives from our previous ordering using a heptagon pattern: place the seven metals in a circle in the sequence of their physical properties, as given above, then start from iron, as having the lowest atomic weight, and score alternately, which gives the ordering by atomic weights (10). 'Classical' Atomic Atomic Metals Weight Number iron 56 26 copper 64 29 silver 108 47 tin 119 50 gold 197 50 mercury 201 80 lead 207 82 A deeper significance of this transform appears within a three-stage process, as follows. One starts off with the days of the week arranged in a circle. The days of the week are named after planetary deities, and the European languages (except German) concur in this respect. Thus Thursday derives from ‘Thor's day', while the French Jeudi is ‘Jupiter's day', the thunder-wielding Thor being a Norse equivalent to Jupiter. Likewise there is an analogy between our Friday, as ‘Freya's day', and Vendredi, ‘Venus' day', with Freya as a Venus-deity, and so forth. .......................... Days, Planets and Metals
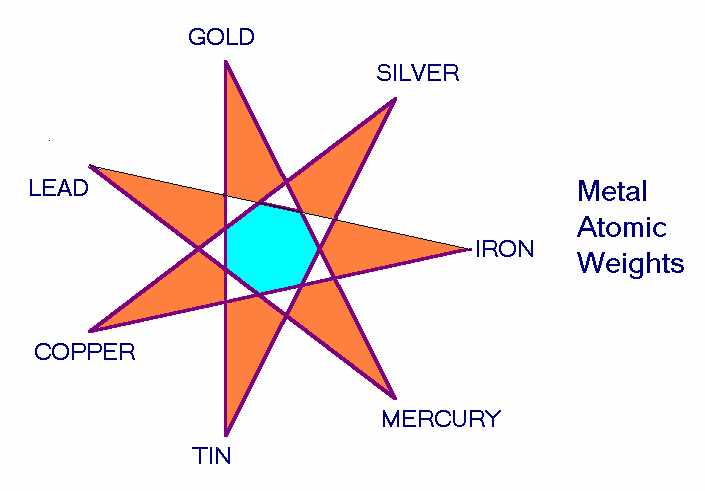
Starting from this circle of the seven days of the week and selecting alternately leads to the ancient, Ptolemaic ordering of the planets. This sequence starts from the Moon, as the sphere closest to the Earth, and ends with Saturn as the furthest of the seven. We saw how this refers to their speeds of motion across the sky, but also to the order of valencies of their corresponding metals, as well as their physical properties. Old books on astronomy used to describe this sevenfold transform, from the Days of Creation sequence, i.e. the seven days of the week, to the old ordering of the planets. They called it, the ‘Hebdomad' (11). Then, early in the twentieth century, the amazing third step of this argument was discerned (12). Selecting every third step around the circle creates a star-heptagon, which gives the ordering by atomic weight or atomic number of the metals! (N.B. This isn’t the same as density). It starts from iron, as having the lowest atomic weight of the classical seven. A sevenfold pattern or mandala starts from the names of sky-gods linked to the days of the week, and then contracts into sequences of physical and chemical properties of the metals. Pelikan seems to have been the first to describe these heptagon-patterns, though not in quite the sequence here presented. In a beautiful and mysterious manner, they link together the concepts of modern chemistry and ancient traditions of the cosmos. From a totally unexpected source, we receive confirmation that there is indeed something special about the ‘seven metals' known to classical antiquity. One American academician, Derek de Solla Price (13), was impressed by the fact that the same geometrical figure, the heptagram, accounted for both the order of the planetary week, and the relationship between the atomic weights of the seven metals and the revolutionary period of their respective planets. He was moved to write: ‘It seems quite plausible that much of astrological theory may rest on just such a basis of figurate rationality rather than upon empirical or special omen lore. In this sense astrology ... developed on a very rational basis, with a figurative theory and the associated symbolism at its centre.'
Atomic Weight Sp.Gravity Melting-Point
Manganese 55 7.4 1200° C
Iron 56 7.9 1528° C
Cobalt 59 8.5 1524° C
Nickel 59 7.7 1500° C
The phrase Pelikan used for these metals was, ‘we have strong reasons to suspect that the iron-Mars impulse cooperated in their formation.' (14) They are more chemically active than iron, their divalent electrode potentials being, manganese +1.2, cobalt +0.28, and nickel +0.25 which is why they were only found long after iron.Praise |